Please click here to get a quote for Fluorescent Peptide Services now!
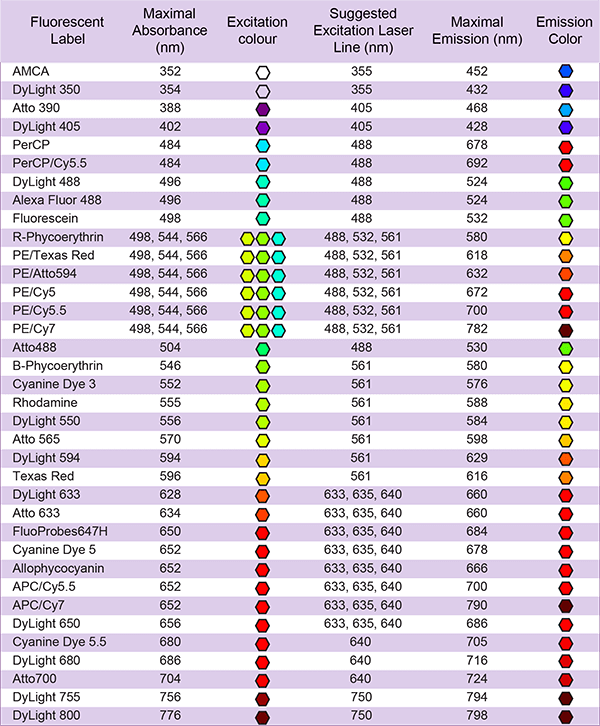
LifeTein provides these fluorescent labelings: FITC, FAM, TAMRA, Cyanine Dye Cy3, Cy3.5, Cy5, Cy5.5, Cy7, Cy7.5, EDANS/Dabcyl, MCA, AZDye, BODIPY FL or Alexa Fluor (Alexa488, Alexa532, Alexa546, Alexa594, Alexa633, Alexa647), ATTO Dyes (Atto465, Atto488, Atto495, Atto550, Atto647), DyLight (DyLight 488, DyLight 550). Contact us if your fluorescent dye is not on the list.
AZDye or Alexa Fluor 647 labeling, Cy3, Cy5, Cy7 Labeling (Structurally, AZDye 647 and Alexa Fluor 647 have the similar fluorophore. Spectrally, these dyes are almost identical: Alexa Fluor 647, AZDye 647, CF 647 Dye, Cy5 Dye, or any other Cyanine5 based fluorescent dyes.)
QSY 9 succinimidyl ester labeling
QSY 9 succinimidyl ester has a strong visible absorption (~560 nm) but no fluorescence. It is used as an acceptor in fluorescence resonance energy transfer (FRET).
Fluorescent dyes are the primary means of labeling biomolecules. The most common such dyes are FITC derivatives, which have a wide range of applications in fluorescence microscopy, flow cytometry, and immunofluorescence-based assays.

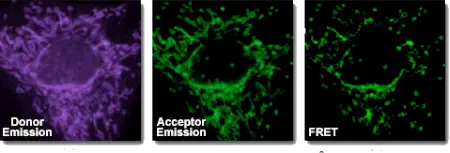
Fluorescence resonance energy transfer (FRET) is a method that allows the distance-dependent interaction between the excited states of two distinct dye-linked molecules to be detected. The excitation is transferred from a donor to an acceptor without emitting a photon. The assay is rapid, highly sensitive, and straightforward to perform. Although it is possible to perform FRET using a single type of dye, the majority of applications require two. A practical measure of FRET efficiency is the distance (Förster-radius) at which the rate of energy transfer equals the rate of donor fluorescence. FRET is particularly appealing for studying co-localization events in biological samples, especially for live-cell imaging.
FRET is used to measure the transfer of energy from the donor that is excited initially (dye 1) to an acceptor (dye 2). The wavelength at which a donor typically emits light, λd, generally overlaps with the absorption wavelength, λa, of the acceptor. The energy transfer occurs in one of two ways, depending on the chemical structure of the acceptor if the donor and acceptor dyes are in close proximity (10–100Å):
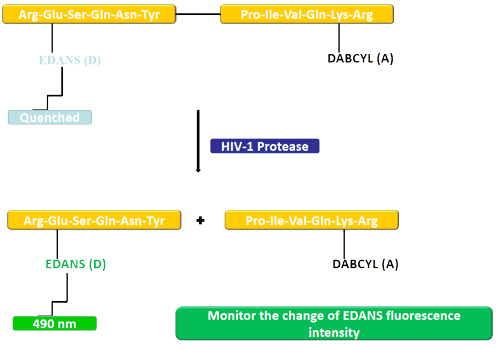
In this example, the donating (EDANS) and accepting groups (DABCYL) are attached to a natural substrate of HIV protease. In an uncleaved substrate, DABCYL quenches EDANS; therefore there is no detectable fluorescence. After the substrate has been cleaved by HIV-1 protease, DABCYL no longer quenches EDANS, allowing the EDANS fluorescence to be detected. Changes in the intensity of EDANS fluorescence can then be monitored to assess the efficiency of protease inhibitors.
FRET peptides can be used to study peptidase specificity because they allow reactions to be monitored continuously, allowing the enzymatic activity to be determined rapidly. The peptide bonds between the donor/acceptor pair can be cleaved, which generates a fluorescent signal to allow nanomolar concentrations of enzyme activity to be measured. When intact, FRET peptides quench internal fluorescence; however, the cleavage of a peptide bond between the donor/acceptor pair releases a fluorescent signal that can be detected continuously, allowing enzyme activity to be quantified.
FRET peptides are used as suitable substrates in many different enzyme studies:
Standard dye combinations used for FRET:
Useful FRET calculator that provides a listing of key FRET pair information: www.fpbase.org/fret/
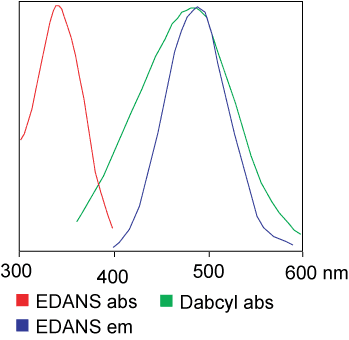
Donor-acceptor pairs capable of quenching using resonance energy transfer in peptide substrates of proteolytic enzymes
|
Wavelengths (nm) |
||
Quencher |
Fluorophore |
Excitation |
Emission |
|
Dabcyl |
Edans |
336 |
490 |
The Forster Critical Distance for Common RET Donor-Acceptor Pairs
Donor |
Acceptor |
Forster Distance |
|
Trp |
Dansyl |
2.1 |
Name |
Ex/Em (nm) |
Emission color |
|---|---|---|
Abz, Anthranilyl, 2-Aminobenzoyl |
320/420 |
|
MCA, 7-Methoxycoumarinyl-4-acetyl |
328/393 |
Blue |
Alexa Fluor: Alexa488, Alexa532, Alexa546, Alexa594, Alexa633, Alexa647 |
650/668 |
Blue,
Red |
FITC (5 or 6 isomer or 5,6-isomers) or BODIPY FL |
494/521 |
Green |
FAM, FITC (5 or 6 isomer or 5,6-isomers) |
494/520 |
Green |
Cy3 |
555/570 |
Yellow |
TAMRA, Carboxytetramethyl rhodamine |
556/563 |
Yellow |
Atto: Atto465, Atto488, Atto495, Atto550, Atto647 |
453/508 |
|
Cy3.5 |
591/604 |
Orange |
Cy3.5 |
591/604 |
Orange |
Texas Red |
589/615 |
Red |
Cy5, Alexa Fluor 647, AZDye 647, CF 647 |
646/662 |
Red |
Cy5.5 |
673/707 |
Near-infrared |
Dylight: DyLight 488, DyLight 550 |
493/518 |
|
Cy7 |
750/773 |
Near-infrared |
Cy7.5 |
788/808 |
Near-infrared |
Reference:
Fluorescent peptide Angiotensin 1-7 (Ang-(1-7)) with FRET pair Abz/[Tyr7(NO2)] was synthesized by LifeTein. This FRET peptide was used to study the Ang-(1-7) endopeptidase in the renal renin angiotensin system. It was found that the endopeptidase is expressed within the renal proximal tubule. It may regulate Ang-(1-7) tone.
... 100 µM Abz-Ang-(1-7)- [Tyr7(NO2)], an internally quenched fluorescent peptide (synthesized by LifeTein, South Plainfield, NJ, USA) for 20 hours or 0.5 nM 125I-(Ang-1-7) for 60 minutes at 37°C ...
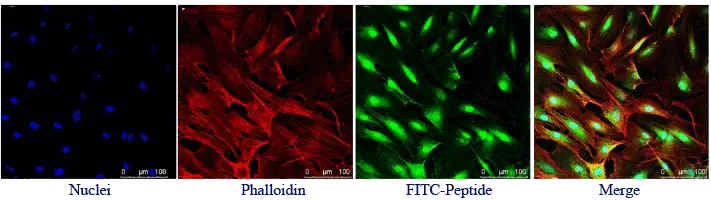
FITC can react extensively with sulfhydryl groups, such as the side chains of reduced cysteine residues.
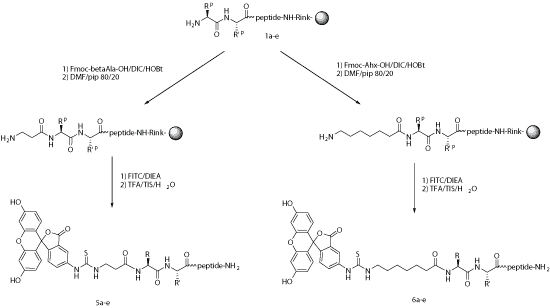
For many applications, fluorescent labels can be introduced during chemical synthesis. After the selective unmasking of a protecting group, FITC might react with either lysine, an ornithine side chain, or with a primary amino group at the N-terminus of the growing peptide. In this latter case, an alkyl spacer such as aminohexanoic acid (Ahx) is introduced between the last amino acid and the thiourea linkage that is generated during the reaction between isothiocyanate and amine.
Under the acidic conditions required for linker cleavage, N-terminal FITC-labeled peptides undergo a cyclization step, which leads to the formation of FITC with the subsequent removal of the last amino acid. This can be avoided when a spacer such as aminohexanoic acid is used, or if the targeted peptide is released from the resin via non-acidic cleavage.
Steric hindrance, which sometimes is the main reason for using Ahx, is no barrier to directly coupling FITC and the peptide. Ahx or b-Ala can be used successfully as spacers to generate FITC-labeled peptides.
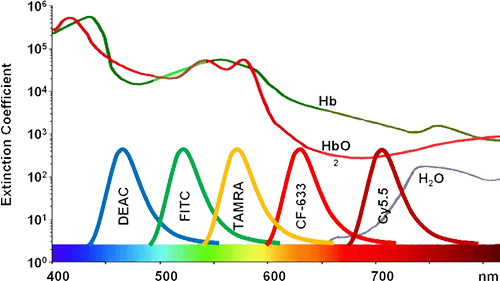
An additional reactive fluorophore, tetramethylrhodamine (TAMRA), also exhibits properties that are not ideal for intracellular reactions. TAMRA-based probes result in the most uniform intracellular distribution of all common FRET probes and have the most efficient diffusion through the cytoplasm. Nevertheless, TAMRA chromophores offer multiple advantages compared with FITC. For example, TAMRA exhibits increased resistance to photobleaching than does FITC. FITC is typically excited at 494 nm and emits fluorescence at 520 nm. In contrast, the maximum absorbance and emission of TAMRA occur at 546 nm and 580 nm, respectively. Importantly, TAMRA contains one extra positive charge compared with, which might increase the interaction between TAMRA and target compounds, and also facilitate binding between the probe and the compound. Similar to Cy3, TAMRA is very stable and bright; therefore, it is an excellent fluorochrome. Importantly, the combination of FITC and TAMRA can be used because of the separation between their respective excitation and emission maxima and because they do not quench each other.
BODIPY FL dye is a bright, photostable, green-fluorescent dye with similar excitation and emission to FITC, FAM or Alexa Fluor 488 dye. This dye is a good choice for microscopy and polarization assays.
Fluorescent dyes such as biotin and FITC can generally be added to either the N- or C-terminus of a peptide. Nevertheless, we recommend adding such modifications to the N terminus because it results in shorter turnaround time, is more likely to be successful, and does not require additional coupling steps. Because peptides are synthesized from the C- to the N-terminus, the addition of an N-terminal modification is the final step in the SPPS protocol.
The majority of commonly used dyes are large aromatic compounds; therefore, the incorporation of these bulky molecules could prevent interactions between the peptide and the dye. This helps the retention of the appropriate peptide conformation and biological activities. We also recommended that clients include a flexible spacer, such as the six-carbon linker Ahx, in their synthetic peptide projects because it increased the stability of the fluorescent label. FITC can also be linked readily to the thiol of cysteine or an amino group of lysine.
| Probe | Ex (nm) | Em (nm) | MW | Notes |
|---|---|---|---|---|
| Methoxycoumarin (MCA) | 360 | 410 | 317 | Succinimidyl ester |
| FITC | 495 | 519 | 389 | pH sensitive |
| X-Rhodamine | 570 | 576 | 548 | |
| Rhodamine B | 570 | 590 | ||
| Cy2 | 492 | 510 | ||
| Cy3 | 550 | 570 | ||
| Cy5 | 650 | 670 |
ATTO Dyes
Atto dyes can be used to replace regular fluorescent dyes. Atto dyes have enhanced photostability and ozone resistance, long signal lifetimes, and reduced background for higher sensitivity. They are ideal for multiplex techniques using visible and near-IR emission wavelengths. For example, Atto 488 is a superior alternative to FITC and Alexa Fluor 488, producing conjugates with more photostability and brighter fluorescence. Atto 550 is an alternative to rhodamine dyes, Cy3, and Alexa Fluor 550, offering more intense brightness and increased photostability.
| Fluorophores | Recommended Atto Dye Alternative |
|---|---|
| Alexa Fluor 488 | Atto 488 |
| FITC | Atto 488 |
| FAM | Atto 488 |
| JOE | Atto 520 |
| TET | Atto 520 |
| Alexa Fluor 532 | Atto 532 |
| HEX™ | Atto 532,Atto Rho6G |
| TAMRA | Atto 550 |
| Cy3 | Atto 550 |
| Cy3.5 | Atto 565 |
| ROX | Atto 565,Atto Rho11 |
| Alexa Fluor 594 | Atto 590,Atto 594 |
| Texas Red | Atto 590 |
| Alexa Fluor 633 | Atto 633,Atto Rho14 |
| Cy5 | Atto 647,Atto 647N,Atto 655 |
| Alexa Fluor 647 | Atto 647,Atto 647N,Atto 655 |
| Cy5.5 | Atto 680,Atto 700 |
| Light source | Main lines (nm) | Recommended Atto dyes |
|---|---|---|
| Mercury arc lamp | 365, 405, 436, 546 | Atto 390, Atto 425, Atto 465, Atto 550, Atto 565 |
| Mercury arc lamp | 577 | Atto 590, Atto Rho101, Atto 594; Atto Rho13, Atto 610, Atto 611x |
| Xenon arc lamp | Continuum and peaks >800 nm | Atto 610, Atto 620, Atto 647, Atto 647N, Atto 655, Atto 680 |
| Halogen lamp | Little UV and violet emission; Higher intensity toward longer wavelengths | Atto 610, Atto 620, Atto 647, Atto 647N, Atto 655, Atto 680 |
| Argon ion laser | 488, 514 | Atto 488, Atto 520, Atto 532, Atto 550 |
| Argon-krypton laser | 488, 514, 647, 676 | Atto 520, Atto 647, Atto 647N, Atto 655, Atto 680 |
| Krypton laser | 647,676 | Atto 647, Atto 647N , Atto 655, Atto Oxa12, Atto 665, Atto 680, Atto 700, Atto 725, Atto 740 |
| He-Ne laser | 633 | Atto Rho14, Atto 633, Atto 647, Atto 647N |
| Nd-NAG laser | 532 | Atto 532, Atto Rho6G, Atto 550, Atto 565, Atto Rho11, Atto Rho 12 |
| Common diode laser | 635, 650, 670 | Atto 633, Atto 647, Atto 647N, Atto 655, Atto 680 |
Förster-radius of selected ATTO-Dye pairs is presented here.

Please click here to see more FAQs
Is a spacer required for fluorescent modification?
How should I dissolve peptides?
How do I choose the best level of peptide purity for my research?
This case study shows the most commonly used approach for FRET assays. The fluorescent dye MCA (methoxycoumarine acetic acid) is incorporated at the N-terminus of a peptide, which was synthesized as a substrate for stromelysin, a matrix metalloprotease. The quencher N-3-(2, 4-dinitrophenyl)-L-2,3-diamino propionyl (DPA) was then incorporated after the enzymatic hydrolysis of the scissile Gly-Leu peptide bond (Knight et al., 1992).
The sequence of the peptide used is as follows:
MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2
The peak absorbance and peak fluorescence of MCA occur at 328 nm and 393 nm, respectively. The DPA group exhibits strong absorption at 363 nm with a prominent shoulder at 410 nm. DPA can quench the fluorescence of MCA because this shoulder overlaps with the fluorescence peak of MCA.
A previous study demonstrated that a 1-μm solution of MCA-Pro-Leu (the product of enzymatic hydrolysis) showed excitation and emission at 328 nm and 393 nm, respectively, and was 130-fold more fluorescent than a comparable solution of MCA-Pro-Leu-Gly-Leu-DPA-Ala-Arg-NH2 (Knight et al., 1992).
Enzymatic hydrolysis of this peptide cause separation of the MCA and DPA groups and a significant increase in MCA fluorescence. This increase in fluorescence can serve as an indicator of the speed of the reaction. This has allowed investigators to establish the values of Kcat/Km of this substrate for several members of the matrix metalloprotease family. This assay was recently used to determine the potency of potential inhibitors of stromelysin through measurement of the effects of the inhibitors on the initial velocity of the enzymatic reaction (Copeland et al., 1995)
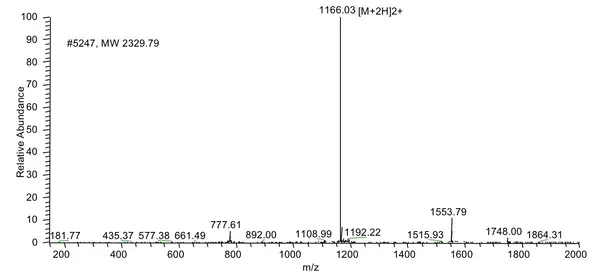
The client requested a very hydrophobic peptide 68 amino acid in length (85% purity) with FITC modification at the N-terminus. The peptide was successfully synthesized in 4 weeks.
HPLC Results:
MS Results: