|
Tobacco Etch Virus (TEV) Protease Recognition Sequence
ENLYFQS |
|
4mg |
|
>95% |
|
LT8226 |
| 2521.74 |
| C106H169N29O40S |
|
(Biotin)-GSGSENLYFQSGS-linker-GSGSKKK
(Biotin)-ENLYFQS-GSGSKK |
|
The peptide sequence ENLYFQ is a recognition sequence for the Tobacco Etch Virus (TEV) protease, which is a highly specific cysteine protease widely used in biotechnology. This enzyme cleaves precisely at the ENLYFQS site, where the scissile bond is between Q (glutamine) and S (serine). Though serine (S) is the most efficient residue in the P1’ position (immediately following the Q), the sequence can also tolerate glycine (G), alanine (A), methionine (M), cysteine (C), or histidine (H) without a significant drop in activity.
TEV protease’s specificity makes it ideal for removing affinity tags like maltose-binding protein (MBP) or poly-histidine tags, which are frequently used in recombinant protein purification. Because of its high precision, TEV protease can minimize unintended cleavage in the target protein. After digestion, the TEV protease itself can be easily removed from the reaction using its 7xHis-tag, which binds to nickel affinity resins, simplifying the purification of the cleaved protein product.
Additionally, engineered versions of TEV protease have been developed to enhance thermal stability and reduce autolysis, which improves its utility in a variety of laboratory conditions. These modifications help to maintain enzymatic activity over a broader range of temperatures, making it suitable for diverse protein purification workflows in research and therapeutic applications?
|
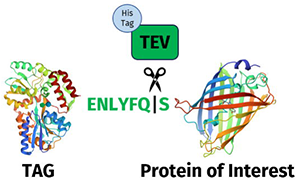
TEV Protease and Its Unique Sequence Specificity:
Tobacco Etch Virus (TEV) protease is a highly sequence-specific cysteine protease that cleaves precisely at the consensus sequence ENLYFQS, where ‘’ marks the peptide bond cleaved between glutamine (Q) and serine (S). This specificity has made TEV protease a powerful tool in biotechnology, particularly for processing recombinant proteins. Its precise cleavage pattern ensures that it removes affinity tags or unwanted sequences without additional non-specific cuts, preserving the integrity of target proteins and providing high reproducibility in various experimental conditions.
Due to its precision, TEV protease is invaluable in protein production, where tags are often used to facilitate purification but must be removed from the final product. TEV’s ENLYFQS recognition site enables researchers to cleave these tags accurately, yielding proteins with native sequences essential for structural and functional studies. Additionally, TEV protease plays a critical role in studying protein-protein interactions by tagging proteins with ENLYFQS sites in biosensors and conducting enzyme assays to investigate molecular mechanisms within cellular environments.
TEV protease’s structural design, particularly its catalytic triad (His46, Asp81, Cys151), enables high selectivity and has inspired extensive research on improving its function. Through techniques like molecular dynamics (MD) simulations, scientists have created modified TEV variants that increase efficiency and reduce self-cleavage tendencies. These studies show how structural adaptations around the active site enhance catalytic function, broadening TEV's use in more demanding biotechnological applications and guiding efforts in enzyme engineering.
The ENLYFQS sequence’s uniqueness and the ongoing innovations in TEV protease design continue to expand its utility across both basic research and therapeutic development, establishing it as a reliable and versatile tool for precise protein processing in laboratory settings.
 https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
 https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873