LifeTein Peptide Conjugates: Click Chemistry
Please click here to get a quote for Peptide Conjugation Service and Click Chemistry now!

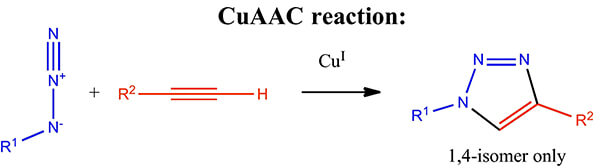
The 2022 Nobel Prize in Chemistry was awarded to Carolyn R. Bertozzi, Morten Meldal, and K. Barry Sharpless for their outstanding contributions to the field of click chemistry—a well-deserved recognition for pioneering a vital and straightforward technique in modern chemistry.
The impact of click chemistry extends across diverse areas of the field, notably in the synthesis of peptides. For instance, LifeTein routinely employs Cu(I)-catalyzed Azide-Alkyne Click Chemistry (CuAAC), Strain-promoted Azide-Alkyne Click Chemistry (SPAAC), and ligation between tetrazine and alkene (trans-Cyclooctene) reactions in its peptide-drug conjugation services. Click chemistry has proven indispensable in linking large and functionalized molecules such as peptides, lipids, and oligonucleotides—a challenging task made feasible by this elegant solution.
LifeTein Conjugation Services
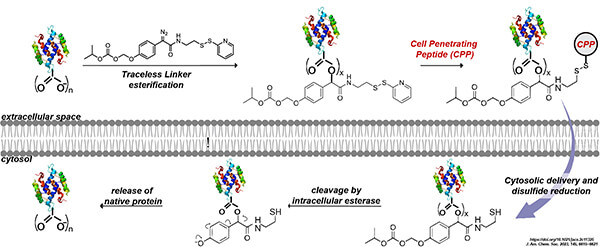
Traceless Protein-CPP Conjugation: LifeTein introduces a versatile strategy for the bioreversible modification of proteins for cellular delivery, incorporating efficient cell-penetrating peptides (CPPs). Our strategy relies on a tricomponent molecule that includes a diazo moiety for the chemoselective esterification of carboxyl groups on the target protein. The linker and CPP will be removed by a self-immolative carbonate group, facilitating esterase-mediated cleavage. This innovative approach offers a traceless modification, ensuring minimal interference and optimal cellular delivery of the protein-CPP conjugate.
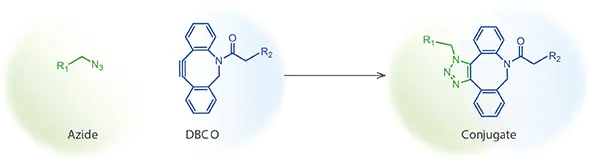
Peptide-Drug Conjugation:LifeTein's Peptide Drug Conjugation encompass a spectrum of applications. The peptide-drug conjugation service offers the development of peptide-drug and antibody-drug conjugates, with successful conjugations involving MMAE, Panobinostat, Tazmetostat, and FK506. Site-specific PEGylation of peptides is facilitated through maleimide or click chemistry, providing versatility for conjugation with proteins, polymers, cholesterol, lipids, DNAs, RNAs, small molecules, or Lipid Nanoparticles
Peptide Pegylation Service: We can provide site-specific PEGylation of peptides using maleimide or click chemistry. Your target can be proteins, polymers, cholesterol, lipids, DNAs, RNAs, small molecules, or nanoparticles. These PEG products are frequently used for conjugation: Cholesterol-PEG-MAL, Biotin-PEG-MAL, DSPE-PEG-MAL, FITC-PEG-MAL, Propargyl-PEG-Maleimide, mPEG-MAL, 4-Arm PEG-MAL, 8-Arm PEG-MAL (MW 1K, 2K, 5K, 10K ). Click for more linkers.
Photo Crosslinking Peptides: We use the photo-labile amino acid p-benzoyl-L-phenylalanine (Bpa) for peptide synthesis and downstream click chemistry to these groups: Functionalized Alkynes, Fluorophore Alkynes/Azides, Nucleoside Azides/Alkynes, Carbohydrate Azides, Organic Azides, PEG Azides, Dibenzocyclooctyne (DBCO), TCO (trans-cycloctene), Tetrazine, or BCN (bicyclo[6.1.0]nonyne).
Oligo-Peptides Conjugation: LifeTein offers DNA-peptide conjugate, RNA-peptide conjugate, Morpholino oligomer peptide, or phosphorodiamidate Morpholino oligomer (PMO) peptide conjugation for cell screening and in vivo studies. The phosphorodiamidate Morpholino oligomer (PMO) contains DNA bases attached to a backbone of methylenemorpholine rings linked through phosphorodiamidate groups.
Peptide–tetrazine conjugate service:
A methyltetrazine moiety that participates in an inverse electron-demand Diels–Alder (IEDDA) reaction with strained alkenes (for example, trans-cyclooctene (TCO) or norbornene) in a copper-free “click” fashion.
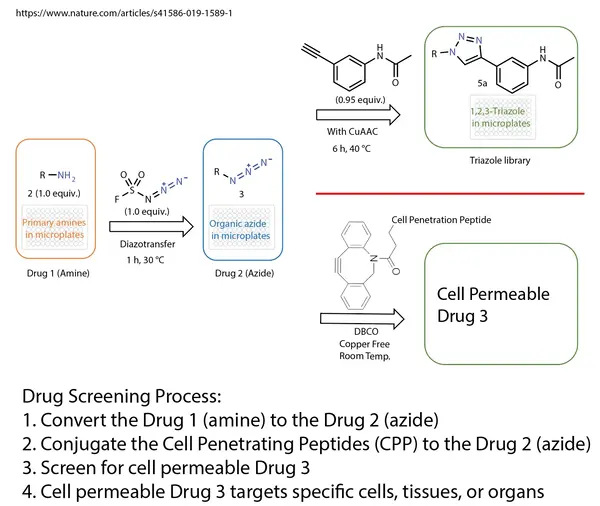
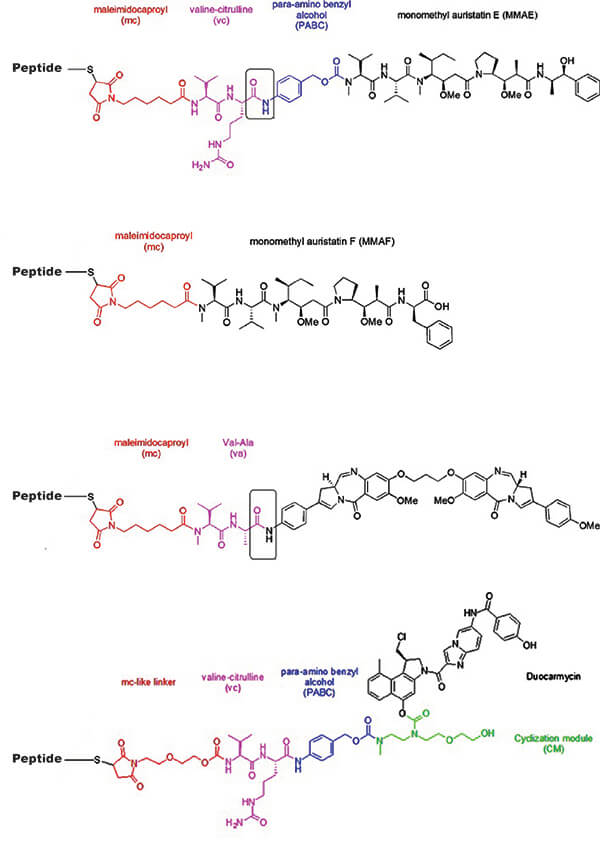
Reference: doi:10.1007/s11095-015-1657-7
Sample Data
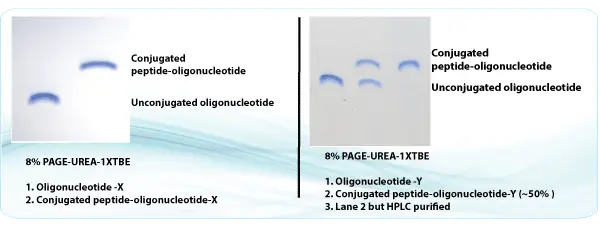
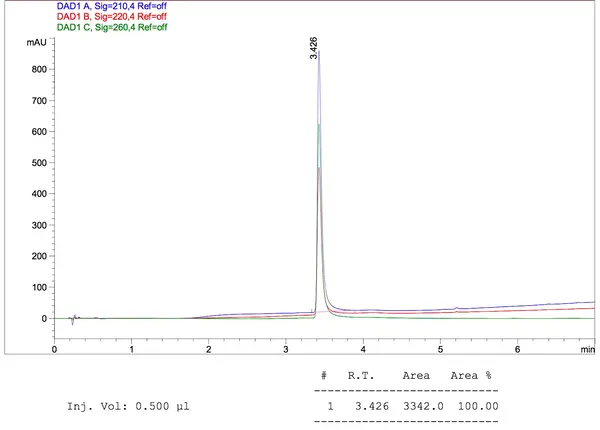
Please click here to get a quote for oligo peptide conjugate service now!







