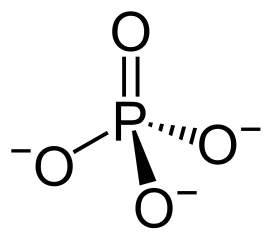
Phosphorylated peptides are a result of a widespread post-translational modification that occurs as a result of esterification of amino acid side chains in peptides. This process involves the addition of a strongly negatively charged phosphate group, thereby altering the protein’s conformation, activity, and ability to interact with other molecules.
Key Takeaways:
- Phosphorylated peptides are a result of a post-translational modification.
- The process involves the addition of a phosphate group to amino acid side chains in peptides.
- This modification alters the protein’s conformation, activity, and ability to interact with other molecules.
- Phosphorylated peptides play an important role in the regulation of many biological processes.
The Importance of Phosphorylated Peptides
Role in Biological Mechanisms
Phosphorylated peptides play an important role in the regulation of many biological processes, such as signaling, gene expression, and cell division. The mechanism of peptide phosphorylation is that the process of transferring the phosphoric acid group of GTP or ATP γ-position to the protein amino acid residue on the basis of kinase catalysis. This occurs mainly on the hydroxyl groups of serine, tyrosine and threonine residue side chains2. It plays an important role in signaling between cells.
Impact on Protein Function
Protein phosphorylation often activates (or deactivates) many enzymes. Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP), the “high-energy” exchange medium in the cell. During aerobic respiration, ATP is synthesized in the mitochondrion by addition of a third phosphate group to adenosine diphosphate (ADP) in a process referred to as oxidative phosphorylation.
Challenges in Studying Phosphorylated Peptides
While protein regions that do not contain post-translational modifications (PTMs) can be rather simply mimicked using peptide libraries, heavily phosphorylated regions are much harder to study using the same tools. The differences between the syntheses of simple mono-, di- and tri-phosphopeptides and the synthesis of multiphosphopeptides are dramatic. Synthesis of multiphosphopeptides requires the insertion of several phosphate groups simultaneously or sequentially into various positions on the peptide in the presence of many other potential modification sites.
Find our list of modifications here.
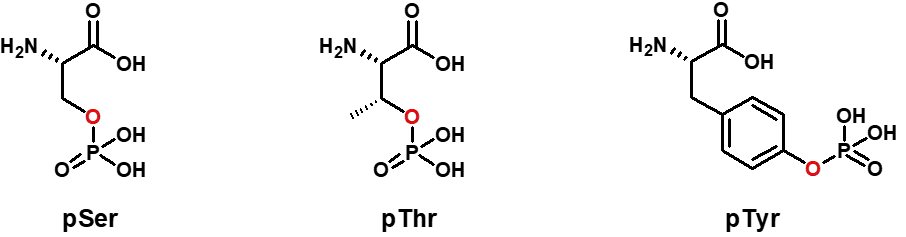
Synthetic Strategies for Phosphorylated Peptides
Traditional Methods
Traditional methods for the synthesis of phosphorylated peptides involve the use of phosphoramidite or phosphotriester chemistry. These methods, while effective, can be time-consuming and require the use of harsh reaction conditions. Additionally, these methods often result in the formation of by-products that can complicate the purification process.
Modern Techniques
Modern techniques for the synthesis of phosphorylated peptides have focused on improving the efficiency and selectivity of the phosphorylation process. One such technique involves the use of solid-phase peptide synthesis (SPPS), which allows for the rapid and efficient synthesis of phosphorylated peptides. This method involves the sequential addition of amino acids to a growing peptide chain attached to a solid support, and is LifeTein’s specialty.
Read more about our solid-phase peptide synthesis here.
Applications of Phosphorylated Peptides
Phosphorylated peptides have a wide range of applications in biological research and medicine. They are often used to study protein-protein interactions, enzyme activity, and cellular signaling pathways. In medicine, phosphorylated peptides have potential applications in developing new therapeutic strategies for diseases such as cancer and neurodegenerative disorders.
Frequently Asked Questions
What are phosphorylated peptides?
- Phosphorylated peptides are peptides that have undergone a post-translational modification involving the addition of a phosphate group to an amino acid residue.
How are phosphorylated peptides synthesized?
- Phosphorylated peptides can be synthesized using traditional methods such as phosphoramidite or phosphotriester chemistry, or modern techniques such as solid-phase peptide synthesis (SPPS).
What are the applications of phosphorylated peptides?
- Phosphorylated peptides have a wide range of applications in biological research and medicine. They are often used as tools for studying protein-protein interactions, enzyme activity, and cellular signaling pathways. In medicine, they have potential applications in the development of new therapeutic strategies for diseases such as cancer and neurodegenerative disorders.