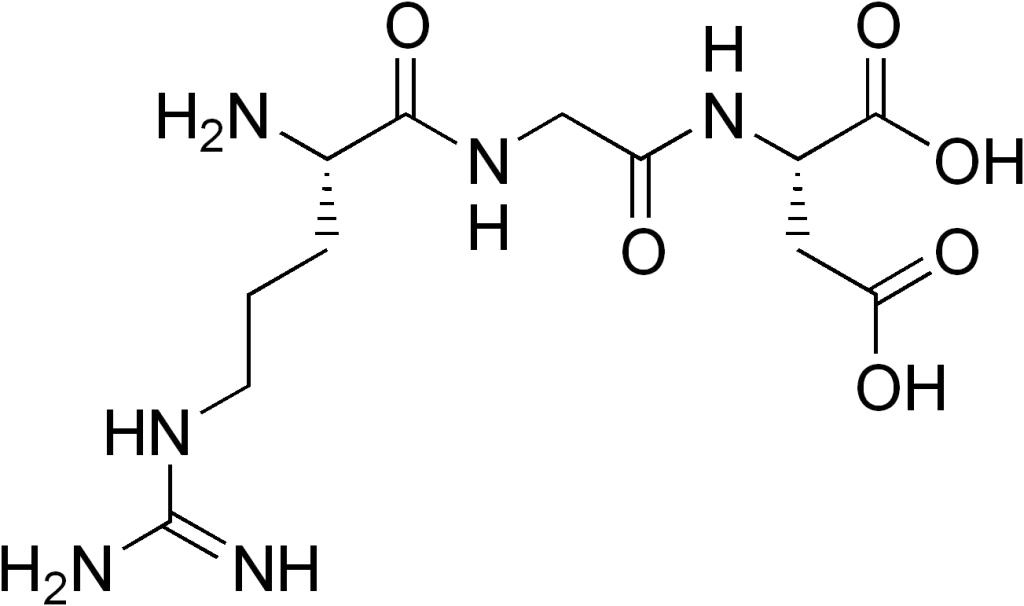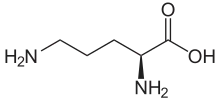
Ornithine, a non-proteinogenic α-amino acid, occupies a unique niche in biochemical pathways due to its critical role in the urea cycle and arginine biosynthesis. Unlike the 20 canonical amino acids encoded by DNA, ornithine is not incorporated into proteins during translation, yet it serves as a central intermediate in nitrogen metabolism and detoxification. This article explores the structural, metabolic, and applied significance of ornithine, highlighting its indispensable contributions to cellular homeostasis and human health.
Key Takeaways
- Ornithine is a non-proteinogenic amino acid central to the urea cycle, enabling ammonia detoxification in mammals.
- It acts as a precursor for arginine, polyamines, and glutamate, influencing processes like cell proliferation and immune function.
- Ornithine transcarbamylase (OTC) deficiency is a rare genetic disorder linked to hyperammonemia, underscoring its metabolic importance.
- Supplementation with ornithine is studied for potential benefits in athletic performance, wound healing, and liver health.
Structural and Biochemical Properties of Ornithine
Non-Proteinogenic Nature
Ornithine is classified as a non-proteinogenic amino acid, meaning it is not directly encoded by the genetic code or incorporated into proteins. Structurally, it resembles lysine but lacks a side-chain methyl group, featuring a four-carbon backbone with a terminal amine group. This configuration allows ornithine to participate in specialized biochemical reactions, particularly in the mitochondria and cytosol.
Role in the Urea Cycle
The urea cycle, a critical pathway in terrestrial vertebrates, relies on ornithine to convert toxic ammonia into urea for excretion. Ornithine combines with carbamoyl phosphate to form citrulline, catalyzed by ornithine transcarbamylase (OTC). This reaction not only mitigates ammonia toxicity but also regenerates ornithine, creating a cyclic process essential for nitrogen balance.
Find custom peptide synthesis with Ornithine here.
Metabolic Pathways Involving Ornithine
The Urea Cycle: A Lifeline Against Ammonia Toxicity
In hepatocytes, ornithine acts as a carrier molecule, shuttling nitrogen through the urea cycle. Excess ammonia from amino acid catabolism is converted into urea via a series of reactions that regenerate ornithine. Disruptions in this cycle—such as OTC deficiency—lead to hyperammonemia, which can cause neurological damage or death if untreated.
Arginine and Polyamine Biosynthesis
Beyond the urea cycle, ornithine serves as a precursor for arginine, a conditionally essential amino acid vital for nitric oxide (NO) production. Additionally, ornithine decarboxylase (ODC) converts ornithine into putrescine, the foundational molecule for polyamines like spermidine and spermine. These compounds regulate DNA stability, apoptosis, and cell proliferation, linking ornithine to broader cellular functions.
Applications of Ornithine in Health and Research
Pharmaceutical and Therapeutic Potential
Ornithine supplementation has been explored for its role in reducing fatigue and enhancing athletic performance by modulating ammonia levels during prolonged exercise. Clinically, ornithine aspartate is used to treat hepatic encephalopathy, leveraging its ability to lower blood ammonia concentrations. Emerging studies also investigate its efficacy in wound healing and muscle recovery post-trauma.
Research Tools and Biochemical Studies
Synthetic ornithine derivatives, such as ornithine hydroxamate, are employed in enzymology to study OTC kinetics and inhibitor interactions. Companies like LifeTein specialize in custom synthesis of ornithine-based peptides and probes, facilitating advanced studies in metabolic disorders and drug discovery.
LifeTein’s Contributions to Ornithine Research
LifeTein, a leader in peptide and amino acid synthesis, offers high-purity ornithine derivatives tailored for research and therapeutic applications. Their expertise in solid-phase peptide synthesis (SPPS) enables the production of ornithine-containing peptides with site-specific modifications, aiding studies on enzyme kinetics and polyamine interactions. Additionally, LifeTein provides fluorescently labeled ornithine analogs for tracking metabolic flux in real-time cellular assays.
Get peptides fast with RUSH synthesis.