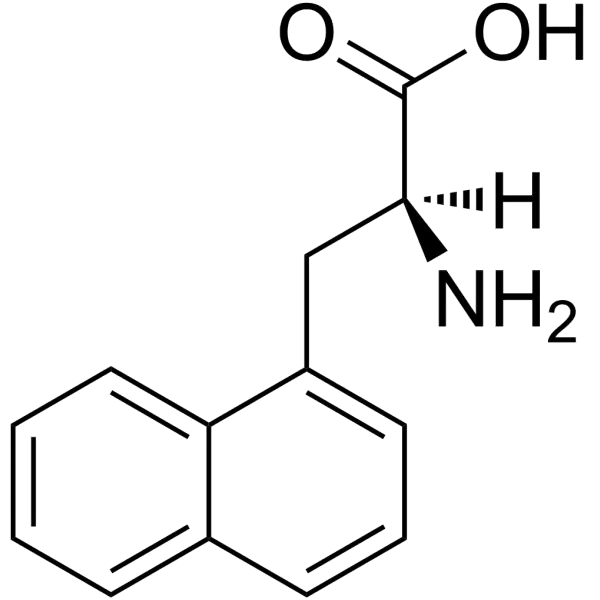
Unusual amino acids represent a fascinating frontier in biochemistry and molecular engineering, offering functionalities beyond the 20 canonical proteinogenic amino acids. Among these, naphthylalanine (Nal) stands out for its unique structural and photophysical properties. Characterized by a naphthalene ring system, this non-natural amino acid exists in two primary isomeric forms: 1-naphthylalanine (1-Nal) and 2-naphthylalanine (2-Nal), distinguished by the attachment position of the naphthyl group to the alanine backbone. Its bulky aromatic side chain enhances hydrophobicity and steric influence, making it invaluable for probing protein folding, receptor-ligand interactions, and fluorescence-based applications. Consequently, Nal has become a cornerstone in peptide engineering and synthetic biology.
Key Takeaways
- Structural versatility: Nal’s isomers (1-Nal and 2-Nal) provide distinct steric and electronic profiles for peptide design.
- Bioconjugation compatibility: Amenable to solid-phase peptide synthesis (SPPS) using Fmoc- or Boc-protected derivatives.
- Research utility: Critical for studying protein interactions, enzyme specificity, and cellular uptake mechanisms.
Structural and Chemical Properties
Molecular Architecture
Naphthylalanine (C₁₃H₁₃NO₂) features a naphthalene moiety fused to the β-carbon of alanine. The 1-Nal isomer (CAS 55516-54-6) attaches the naphthyl group at the 1-position, while 2-Nal (CAS 58438-03-2) attaches it at the 2-position. This difference significantly impacts their chemical behavior: 1-Nal exhibits greater steric hindrance and a higher melting point compared to 2-Nal. Both isomers are soluble in organic solvents (e.g., DMSO, chloroform) but exhibit limited water solubility, necessitating tailored buffer conditions for biological assays.
Spectral and Electronic Traits
The extended π-conjugation of the naphthalene ring confers intrinsic fluorescence, with absorption/emission profiles suitable for UV-Vis detection. Furthermore, its hydrophobicity enhances membrane permeability, making it ideal for cell-penetrating peptide designs.
Find out more about peptide synthesis here.
Applications in Biochemical Research
Peptide Therapeutics and Drug Design
Nal’s hydrophobicity and stability enhance peptide-drug pharmacokinetics. It is incorporated into peptidomimetics targeting enzymes or receptors. Notably, Nal derivatives bind the Salmonella typhimurium OppA transporter, revealing pathways for antimicrobial development.
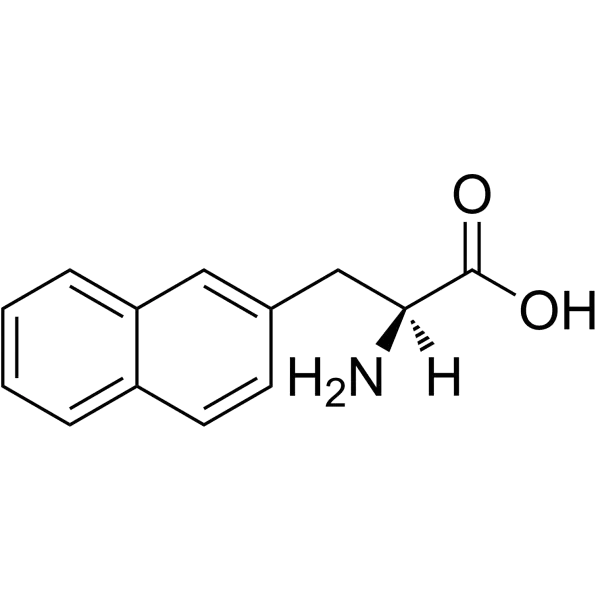
Synthesis and Conjugation Methods
Solid-Phase Peptide Synthesis (SPPS)
Nal is incorporated into peptides using Fmoc- or Boc-protected precursors (e.g., Fmoc-1-Nal-OH, CAS 96402-49-2). LifeTein’s SPPS protocols achieve high-purity (>95%) Nal-labeled peptides, even for highly hydrophobic sequences up to 68 amino acids. Critical considerations include:
- Spacer integration: Aminohexanoic acid (Ahx) spacers prevent steric hindrance during dye conjugation.
- Orthogonal protection: Boc groups preserve side-chain functionality during fluorescent labeling.
Find out about high-speed RUSH synthesis.
Future Directions
Ongoing innovations include genetic code expansion to incorporate Nal in vivo via stop codon suppression. Additionally, multiphoton FRET using Nal’s UV-shifted spectra could enable deeper tissue imaging.
Frequently Asked Questions (FAQ)
What distinguishes 1-Nal from 2-Nal?
The attachment position of the naphthyl group: 1-Nal links at the naphthalene’s 1-position, causing greater steric hindrance, while 2-Nal links at the 2-position, offering milder steric effects 48.
Why use Nal instead of phenylalanine in peptide design?
Nal’s larger aromatic surface enhances hydrophobic interactions and fluorescence quenching efficiency, improving sensitivity in FRET and protein-binding studies 39.
Is Nal suitable for cell-penetrating peptides (CPPs)?
Absolutely. Its hydrophobicity enhances membrane permeability, and LifeTein couples it to TAT or R8 CPPs for intracellular delivery studies.
