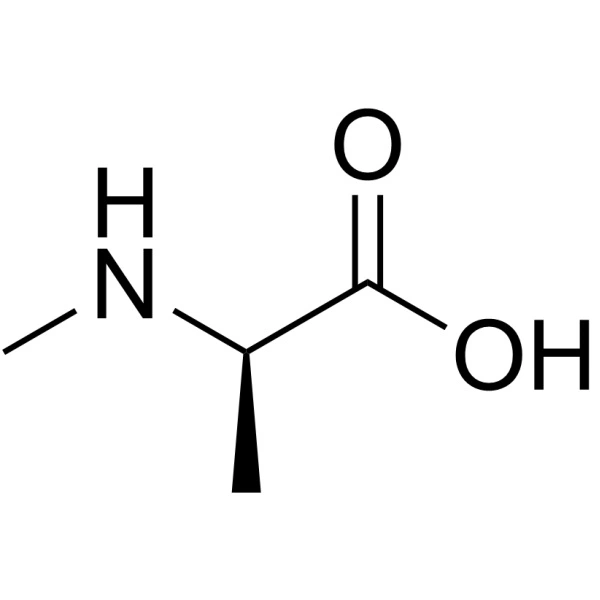
N-methylation, the addition of a methyl group to the amide nitrogen of a peptide backbone, is a powerful synthetic modification for altering a peptide’s fundamental properties. This strategic alteration introduces significant steric hindrance and conformational constraints, which directly influence the peptide’s stability, solubility, and interaction with its environment. The decision to incorporate N-methylated amino acids is not one to be taken lightly; it requires a systematic evaluation of the peptide’s intended application, its sequence, and the synthetic challenges involved.
Key Takeaways
- N-methylation enhances metabolic stability by shielding peptide bonds from proteolytic enzymes.
- The modification improves membrane permeability by reducing the peptide’s hydrogen-bonding capacity.
- Methylation introduces significant synthetic complexity, requiring specialized protocols to avoid low yields and racemization.
- The decision hinges on balancing desired stability and permeability gains against potential losses in solubility and biological activity.
- A residue-specific analysis is critical, as the impact of methylation is highly dependent on its position within the sequence.
Core Principles of N-Methylated Peptides
Structural and Energetic Consequences
The primary effect of N-methylation is the introduction of a methyl group on the peptide bond nitrogen. This adds steric bulk that restricts the conformational freedom of the peptide backbone, potentially stabilizing specific turns or helical structures. From an energetic perspective, it removes a hydrogen bond donor, which significantly increases the lipophilicity of the molecule. This reduction in overall polarity is a key factor in altering the peptide’s behavior in different environments.
Find more peptide modifications here.
Decision Framework: When to Use Methylated Peptides
Objective-Driven Justification
The initial decision must be driven by a clear primary goal. Methylation is strongly justified when the paramount requirements are for enhanced resistance to enzymatic degradation or improved passive diffusion across hydrophobic barriers. If the peptide’s application does not demand exceptional stability or membrane permeability, the complications of methylation may be unnecessary.
Sequence and Residue Analysis
A critical step is a residue-by-residue evaluation. Methylation is most effectively applied to peptide bonds known to be susceptible to proteolytic cleavage, thereby conferring site-specific stability. Methylation should be avoided at residues involved in crucial binding interactions, as the steric bulk can disrupt affinity.
Synthetic Feasibility Assessment
The incorporation of N-methylated amino acids presents notable synthetic challenges. These residues exhibit lower reactivity during standard solid-phase peptide synthesis (SPPS) coupling steps, often leading to incomplete reactions and difficult-to-purify side products. Researchers must assess whether they or their synthesis provider possesses the expertise to overcome these hurdles, which may involve specialized coupling reagents and extended reaction times.
Functional Impacts to Consider
Enhancement of Metabolic Stability
The steric shield provided by the methyl group physically blocks access to proteolytic enzymes. By strategically methylating bonds identified as labile sites, one can dramatically increase the peptide’s longevity in biological systems, a crucial factor for any application requiring prolonged activity.
Alteration of Membrane Permeability
The reduction in hydrogen bond donors decreases the energetic penalty of moving the peptide from an aqueous solution into the lipid bilayer of a cell membrane. This directly translates to improved passive diffusion, making methylation a key strategy for designing peptides that need to traverse cellular barriers effectively.
Potential Impact on Solubility and Aggregation
The increase in lipophilicity can be a double-edged sword. While beneficial for permeability, it often leads to a decrease in aqueous solubility. This can complicate handling, purification, and formulation, potentially leading to issues with peptide aggregation that must be managed through solvent selection or buffer optimization.
Synthesis and Handling Considerations
Navigating Synthetic Complexities
The synthesis of N-methylated peptides requires specialized approaches. The steric hindrance of the methyl group necessitates the use of powerful coupling agents and optimized conditions to achieve efficient bond formation. Providers like LifeTein employ advanced SPPS protocols specifically designed to handle the low nucleophilicity of N-methylated amino acids, minimizing the risk of deletion sequences and epimerization.
Analytical Verification and Characterization
Confirming the successful incorporation and correct structure of a methylated peptide is essential. Analytical techniques such as mass spectrometry are used to verify the expected mass increase (+14 Da per methylation). Furthermore, reversed-phase HPLC typically shows a longer retention time for methylated peptides due to their increased hydrophobicity, providing a key purity and identity check.
Find out more about peptide synthesis here.
Frequently Asked Questions (FAQ)
How does N-methylation differ from O-methylation?
These are distinct modifications. N-methylation targets the amide nitrogen in the peptide backbone, directly influencing backbone flexibility and hydrogen bonding. O-methylation, in contrast, targets the oxygen in side-chain carboxyl groups (e.g., in aspartic or glutamic acid) or hydroxyl groups (e.g., in serine, threonine), primarily altering side-chain polarity and charge.
Can methylation be applied to any position in a peptide sequence?
While technically possible, the effect is highly position-dependent. Methylation is generally well-tolerated in flexible loop regions or to stabilize beta-turns. It is often problematic when applied to residues that are part of a well-defined secondary structure like an alpha-helix, as it can disrupt stabilizing hydrogen-bonding patterns.
What is the primary synthetic challenge when working with N-methylated amino acids?
The main challenge is their low nucleophilicity. The methyl group sterically hinders the nitrogen atom, making it less reactive during the coupling step in solid-phase synthesis. This requires the use of highly efficient coupling reagents, elevated temperatures, or longer reaction times to achieve complete incorporation, all of which can increase the risk of side reactions.
How does methylation affect the final yield and purity of a synthetic peptide?
Incorporating N-methylated amino acids almost invariably lowers the final crude yield and purity compared to a standard peptide sequence. The difficulties in achieving complete coupling lead to a higher proportion of deletion sequences, necessitating more rigorous purification protocols, such as preparatory HPLC, to isolate the correct product.
References:
Hartel, N. G., Chew, B., Qin, J., Xu, J., & Graham, N. A. (2019). Deep Protein Methylation Profiling by Combined Chemical and Immunoaffinity Approaches Reveals Novel PRMT1 Targets. Molecular & Cellular Proteomics, 18(11), 2149–2164. https://doi.org/10.1074/mcp.ra119.001625
Hart-Smith, G., Yagoub, D., Tay, A. P., Pickford, R., & Wilkins, M. R. (2016). Large Scale Mass Spectrometry-based Identifications of Enzyme-mediated Protein Methylation Are Subject to High False Discovery Rates. Molecular & Cellular Proteomics, 15(3), 989–1006. https://doi.org/10.1074/mcp.m115.055384
Janssens, Y., Wynendaele, E., Vanden Berghe, W., & De Spiegeleer, B. (2019). Peptides as epigenetic modulators: therapeutic implications. Clinical Epigenetics, 11(1). https://doi.org/10.1186/s13148-019-0700-7
Räder, A. F. B., Reichart, F., Weinmüller, M., & Kessler, H. (2018). Improving oral bioavailability of cyclic peptides by N-methylation. Bioorganic & Medicinal Chemistry, 26(10), 2766–2773. https://doi.org/10.1016/j.bmc.2017.08.031
