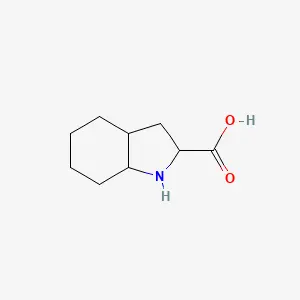
Octahydroindole-2-carboxylic acid (Oic) is a prominent non-proteinogenic, bicyclic amino acid that has become an indispensable tool in advanced peptide design and peptidomimetic chemistry. As a conformationally constrained analogue of proline, Oic introduces significant backbone rigidity and enhanced lipophilicity when incorporated into peptide sequences. These properties are strategically employed to overcome major limitations of therapeutic peptides, such as poor metabolic stability and low bioavailability, by stabilizing specific secondary structures and improving membrane permeability. Consequently, Oic serves as a critical building block in pharmaceuticals, notably in antihypertensive drugs like perindopril and trandolapril, and in clinical-stage compounds for conditions ranging from hereditary angioedema to neurodegenerative diseases.
Key Takeaways
- Conformational Constraint: Oic’s bicyclic structure imparts significant rigidity to the peptide backbone, effectively stabilizing turns and helices and drastically reducing conformational flexibility.
- Enhanced Lipophilicity: The fused cyclohexane ring increases the hydrophobic character of the amino acid, which can improve a peptide’s passive membrane permeability and overall bioavailability.
- Stereochemical Complexity: With three stereogenic centers, Oic has eight possible stereoisomers. The (2S,3aS,7aS)-isomer (L-Oic) is the most prevalent in drug applications, with each isomer offering unique conformational properties.
- Synthetic Accessibility: Oic is used in peptide synthesis via commercially available, orthogonally protected derivatives like Fmoc-L-Oic-OH and Boc-L-Oic-OH, which are compatible with standard solid-phase peptide synthesis (SPPS) protocols.
- Proteolytic Stability: Peptides incorporating Oic exhibit increased resistance to enzymatic degradation, as the rigid structure and non-natural character hinder protease recognition and cleavage.
Chemical Structure and Fundamental Properties
Octahydroindole-2-carboxylic acid features a bicyclic system comprising a proline-like pyrrolidine ring fused to a cyclohexane ring. This structure classifies it as a bicyclic proline analogue. The complete saturation of the system (octahydro-) contributes to its high lipophilicity compared to standard amino acids.
A key feature of Oic is its stereochemical complexity. The molecule possesses three chiral centers (at the 2, 3a, and 7a positions), leading to eight possible stereoisomers. The specific (2S,3aS,7aS) configuration, known as L-Oic, is the isomer most commonly employed in pharmaceutical and peptide research due to its commercial availability and proven utility in bioactive compounds. The stereochemistry at these centers critically influences the three-dimensional orientation of the cyclohexane ring, which in turn dictates the conformational impact Oic exerts on a peptide chain.
Find out more about peptide synthesis here.
Conformational Impact on Peptide Structure
Induction of Backbone Rigidity
The primary structural effect of incorporating Oic is the severe restriction of the φ and ψ backbone dihedral angles at the site of incorporation. This backbone rigidity reduces the entropic penalty upon binding to a target and helps pre-organize the peptide into a bioactive conformation.
Stabilization of Polyproline II Helices
Research has demonstrated that oligomers of Oic spontaneously form stable polyproline type II (PPII) helices, which are extended, left-handed helical structures. The fused cyclohexane ring in a chair conformation anchors the pyrrolidine ring in an exo puckering, which strongly favors the trans configuration of the preceding amide bond. This preference propagates through the chain, leading to a cooperative stabilization of the entire PPII helix. This makes Oic an ideal building block for constructing stable, hydrophobic PPII scaffolds, which are relevant in molecular recognition and biomaterial science.
Promotion of Beta-Turns
In shorter peptide sequences, Oic is exceptionally effective at nucleating and stabilizing beta-turn structures, particularly type II’ β-turns. Its constrained geometry perfectly accommodates the *i+1* or *i+2* position of a turn, making it a valuable tool for cyclizing peptides or mimicking surface loops of proteins in drug design.
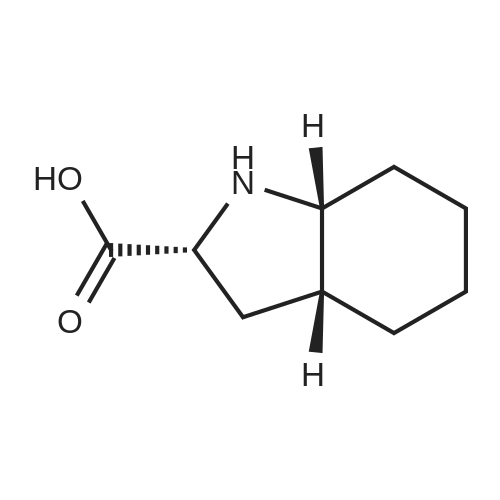
Synthesis and Incorporation into Peptides
Synthetic Routes to Oic
The synthesis of enantiomerically pure Oic, especially non-commercial stereoisomers, presents a considerable challenge due to its three stereocenters. Methodologies reported in the literature include:
- Stereoselective synthesis from chiral precursors.
- Diastereomeric resolution of racemic mixtures via salt formation or chromatography.
- Epimerization and selective functionalization strategies, such as the formation of a trichloromethyloxazolidinone derivative to separate epimers.
Industrial routes, as detailed in patents for drugs like trandolapril, often involve multi-step sequences starting from materials like L-serine or indoline-2-carboxylic acid.
Use in Solid-Phase Peptide Synthesis (SPPS)
For peptide chemists, Oic is readily incorporated using standard Fmoc-SPPS or Boc-SPPS strategies. The amino acid is commercially available in forms suitable for these techniques:
These derivatives ensure efficient and selective incorporation into growing peptide chains on automated synthesizers.
Applications in Pharmaceutical and Peptide Science
Oic’s unique properties have led to its successful integration into several high-profile therapeutic agents:
- Antihypertensive Drugs: Oic is the key dipeptide mimic in perindopril and trandolapril, both angiotensin-converting enzyme (ACE) inhibitors. Its rigid structure is critical for potent enzyme binding.
- Bradykinin B2 Receptor Antagonists: The drug icatibant (HOE 140), used to treat hereditary angioedema, contains Oic as a substitute for proline, conferring potent antagonism and high metabolic stability.
- Enzyme Inhibitors: Oic-based compounds like S 17092 are potent inhibitors of prolyl oligopeptidase (POP), a target for cognitive disorders, showcasing their utility in neuropharmacology.
- Peptidomimetic Design: Beyond direct incorporation, Oic serves as a versatile core structure for designing quaternary amino acid derivatives and other functionalized scaffolds with potential applications in treating joint cartilage damage and as antithrombotic agents.
Find out about high-speed RUSH synthesis.
Frequently Asked Questions (FAQ)
What is the main advantage of using Oic over proline in a peptide?
While both induce conformational constraint, Oic provides significantly greater backbone rigidity and lipophilicity due to its fused, saturated bicyclic structure. This often translates to superior proteolytic stability and enhanced bioavailability in peptide-based therapeutics.
Can Oic be used in standard automated peptide synthesizers?
Yes, absolutely. The commercially available Fmoc-L-Oic-OH and Boc-L-Oic-OH derivatives are fully compatible with standard solid-phase peptide synthesis (SPPS) protocols and coupling reagents, allowing for seamless integration into automated synthesis workflows.
Why is the stereochemistry of Oic so important?
The three-dimensional shape of Oic, dictated by its three stereocenters, determines how it influences peptide folding. Different stereoisomers will project the fused cyclohexane ring in distinct spatial orientations, which can either stabilize or destabilize a desired peptide conformation and dramatically affect binding to a biological target.
Is Oic a natural amino acid?
No, octahydroindole-2-carboxylic acid is a non-proteinogenic, synthetic amino acid. It is not encoded by DNA and is not found in naturally occurring ribosomal proteins, though motifs similar to its structure exist in some complex natural products like aeruginosins.
Sayago, F. J., Isabel Calaza, M., Jiménez, A. I., & Cativiela, C. (2008). Versatile methodology for the synthesis and α-functionalization of (2R,3aS,7aS)-octahydroindole-2-carboxylic acid. Tetrahedron, 64(1), 84–91. https://doi.org/10.1016/j.tet.2007.10.095
Sayago, F. J., Jiménez, A. I., & Cativiela, C. (2007). Efficient access to N-protected derivatives of (R,R,R)- and (S,S,S)-octahydroindole-2-carboxylic acid by HPLC resolution. Tetrahedron: Asymmetry, 18(19), 2358–2364. https://doi.org/10.1016/j.tetasy.2007.09.006
