Keyhole limpet hemocyanin (KLH) is a well-established cornerstone in the generation of peptide-specific antibodies. As a large, highly immunogenic carrier protein sourced from the marine mollusk Megathura crenulata, its primary function is to provide the necessary T-cell help that small, weakly immunogenic peptide antigens lack on their own. The covalent conjugation of your peptide to KLH is often the decisive step in transforming a simple sequence into a potent immunogen capable of eliciting a robust and high-titer antibody response. However, this strategy is not universally optimal; a successful outcome hinges on understanding the benefits, potential pitfalls, and key alternatives before proceeding.
Key Takeaways
- KLH is a powerful immunogenic carrier that provides T-cell epitopes, essential for generating strong, class-switched antibody responses against small peptides.
- The primary goal of conjugation is to enhance immunogenicity. Keyhole limpet hemocyanin has been demonstrated as an optimal carrier, significantly outperforming other proteins in eliciting peptide-specific antibodies in comparative studies.
- A significant drawback is the “carrier effect,” where the immune system can disproportionately target KLH-derived epitopes or terminal peptide “neo-epitopes,” potentially reducing the yield of antibodies against the core peptide of interest.
- Strategic peptide design is critical. LifeTein recommends targeting solvent-exposed, flexible regions (often C- or N-terminal), using sequences of 8-20 amino acids, and managing hydrophobicity for solubility.
- A key alternative is the Multiple Antigenic Peptide (MAP) system, which uses a branched lysine core to present multiple peptide copies without a biological carrier, thereby avoiding anti-carrier antibodies and focusing the response on the peptide.
What is KLH and Why is it Used?
KLH is a high-molecular-weight, copper-containing glycoprotein renowned for its strong immunogenicity and low toxicity in animals and humans. Its effectiveness stems from its size and complex structure, which are rich in foreign epitopes that can be recognized by helper T cells of the host immune system. Peptides, especially those shorter than 20 amino acids, are typically too small to be efficiently recognized by B cells and lack the necessary T-cell epitopes to stimulate a mature, high-affinity IgG response. By conjugating the peptide to Keyhole limpet hemocyanin, you effectively “piggyback” its presentation onto a protein that efficiently engages both arms of the adaptive immune system, leading to enhanced antibody titers and isotype maturation (e.g., increased IgG1).
The Compelling Benefits of KLH Conjugation
Superior Immunogenic Potency
Extensive research validates KLH’s role as the benchmark carrier. A pivotal study comparing carrier proteins for cancer antigen vaccines concluded that the covalent attachment to KLH was optimal for inducing potent antibody responses. Recent methodologies further leverage KLH’s potency by combining it with rational peptide sequence optimization, demonstrating that KLH-conjugated, engineered peptides elicit stronger antibody titers and improved affinity against native target sequences.
Find more peptide conjugation here.
Proven Conjugation Chemistry
Reliable, kit-based methods exist for conjugating peptides to KLH. The two most common strategies are:
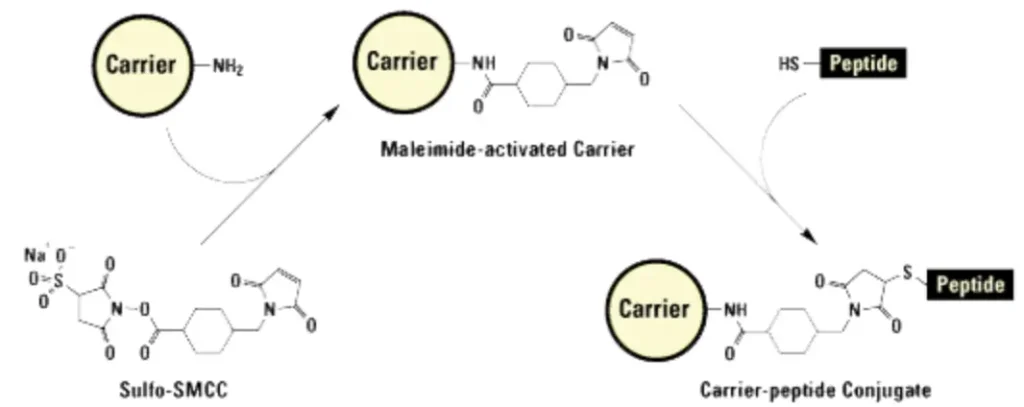
- Maleimide Chemistry: Used for peptides with a terminal cysteine residue. The thiol group of cysteine forms a stable thioether bond with a maleimide-activated KLH molecule.
- Carbodiimide (EDC) Chemistry: Used to conjugate peptides via carboxyl-to-amine crosslinking, typically targeting the N-terminus or lysine side chains of the peptide to lysines on KLH.
These standardized protocols yield consistent conjugation efficiencies, allowing for predictable immunogen preparation.
Critical Considerations and Potential Drawbacks
The Carrier-Specific Response and Neo-Epitope Problem
A major consideration is that the immune system will also generate a vigorous response against KLH itself. This “carrier effect” is not inherently problematic but must be accounted for in assay design. More importantly, research indicates that antibodies raised against peptide-KLH conjugates can be disproportionately directed against the terminal amino acids of the peptide (the linkage region), creating “neo-epitopes” not present in the native, full-length protein. This can result in antisera with poor recognition of the target protein, as the most immunogenic part of the immunogen (the peptide terminus) is irrelevant to the final application.
Solubility and Handling Challenges
Keyhole limpet hemocyanin is notorious for its limited solubility, which can complicate conjugation and handling. While commercial formulations like PEGylated KLH improve solubility, this adds an extra layer of complexity. Furthermore, the large size of the KLH-peptide conjugate can sometimes cause steric hindrance, potentially masking the very epitope you aim to target, especially if it is internal rather than terminal.
Making the Decision: A Framework for Your Project
The choice to use KLH should be guided by your specific experimental goals and the nature of your peptide.
The Multiple Antigenic Peptide (MAP) Alternative
A powerful alternative to carrier conjugation is the Multiple Antigenic Peptide (MAP) system. This involves synthesizing your peptide on a branched lysine core, creating a macromolecule where the peptide itself constitutes up to 95% of the mass. MAPs are intrinsically immunogenic due to their size and high epitope density, requiring no foreign carrier protein. This eliminates the anti-KLH response and focuses the immune system entirely on the peptide antigen, which can be advantageous for generating highly specific antibodies.
Find out more about peptide synthesis here.
Frequently Asked Questions (FAQ)
Is KLH safe to use for immunization?
Yes. KLH is widely used in both research and clinical settings due to its high immunogenicity and low toxicity. It has been employed in human cancer vaccine trials and as an immunomodulator for decades.
How many peptides should I conjugate to each KLH molecule?
A high ratio is standard. Protocols often use a molar ratio of 80:1 (peptide:KLH) or similar to ensure the carrier surface is densely decorated with hapten, maximizing B-cell receptor engagement. Commercial activation kits are optimized to provide a high number of conjugation sites per KLH molecule.
Can I use something other than KLH as a carrier?
Yes, other proteins like bovine serum albumin (BSA) or ovalbumin (OVA) are common. However, KLH is generally preferred for primary immunization due to its superior foreignness and immunogenicity. BSA or OVA are often used as coating antigens in assay development to avoid detecting anti-carrier antibodies from the serum.
Where can I get help designing my peptide antigen and conjugation strategy?
Specialized peptide service providers like LifeTein offer free bioinformatics tools and expert support for peptide antigen design, considering factors like solubility, hydrophilicity, and conjugation site selection to maximize your chances of success.
References:
Chen, C.-H., Chiu, Y.-C., Huang, K.-Y., Huang, H.-H., Kuo, T.-W., Liu, Y.-C., Kao, H.-J., Yu, C.-L., Weng, S.-L., & Liao, K.-W. (2025). A Reproducible Sequence-Level Strategy to Enhance Peptide Immunogenicity While Preserving Wild-Type Epitope Recognition. Antibodies, 14(4), 106. https://doi.org/10.3390/antib14040106
Aarntzen, E. H. J. G., de Vries, I. J. M., Göertz, J. H., Beldhuis-Valkis, M., Brouwers, H. M. L. M., van de Rakt, M. W. M. M., van der Molen, R. G., Punt, C. J. A., Adema, G. J., Tacken, P. J., Joosten, I., & Jacobs, J. F. M. (2012). Humoral anti-KLH responses in cancer patients treated with dendritic cell-based immunotherapy are dictated by different vaccination parameters. Cancer Immunology, Immunotherapy, 61(11), 2003–2011. https://doi.org/10.1007/s00262-012-1263-z
Kim, S. K., Ragupathi, G., Cappello, S., Kagan, E., & Livingston, P. O. (2000). Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1–KLH and GD3–KLH conjugates. Vaccine, 19(4–5), 530–537. https://doi.org/10.1016/s0264-410x(00)00195-x
Pon, R., Marcil, A., Chen, W., Gadoury, C., Williams, D., Chan, K., Zhou, H., Ponce, A., Paquet, E., Gurnani, K., Chattopadhyay, A., & Zou, W. (2020). Masking terminal neo-epitopes of linear peptides through glycosylation favours immune responses towards core epitopes producing parental protein bound antibodies. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-75754-7
Oyelaran, O., & Gildersleeve, J. C. (2010). Evaluation of human antibody responses to keyhole limpet hemocyanin on a carbohydrate microarray. PROTEOMICS – Clinical Applications, 4(3), 285–294. https://doi.org/10.1002/prca.200900130
