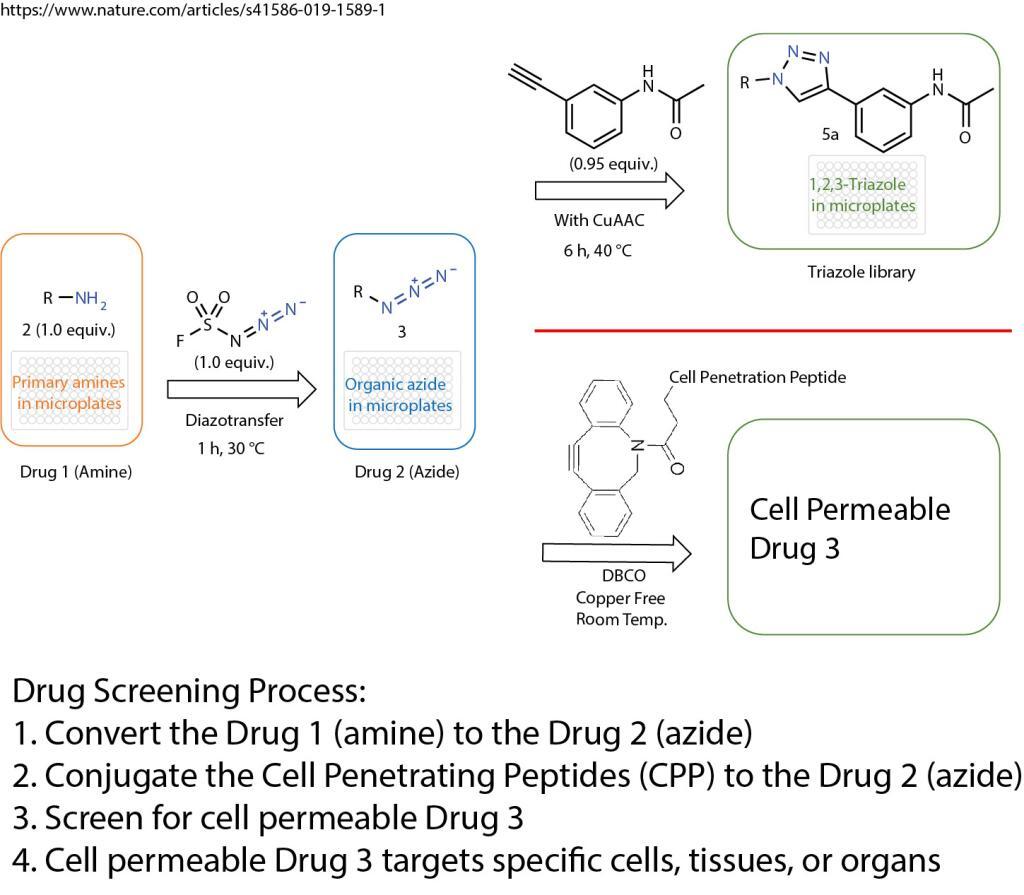
A click chemistry was reported about the formation of azides from primary amines. This powerful tool enables the reaction of just one equivalent of a simple diazotizing species, and fluorosulfuryl azide (FSO2N3), for the preparation of over 1,200 azides on 96-well plates in a safe and practical manner. This method greatly expands the number of accessible azides and 1,2,3-triazoles because the primary amine is one of the most abundant functional groups in small compounds, proteins and antibodies.
Formation of Azides From Primary Amines
The method opens the door for numerous applications in drug screening and discovery. The cell penetration peptides can be easily introduced to conjugate with any azide containing drugs, compounds, antibodies, or proteins.
The cell penetration peptides (CPPs) are capable of delivering biologically active cargo to the cell interior. The desired therapeutic cargo could be attached to a CPP using the copper free click chemistry and then delivered to an intracellular target, thereby overcoming the entry restrictions set by the plasma membrane.
Our Services:
Custom Peptide Synthesis Services
Other Posts:
Smaller Ions Stabilize β-sheets
