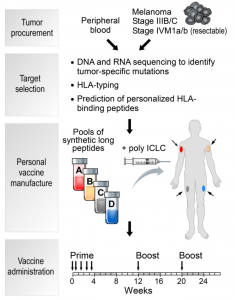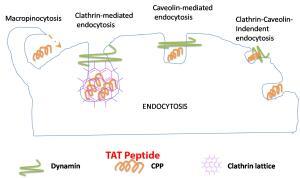
Endocytosis TAT Peptide
Nuclear translocation of annexin A1 (ANXA1) has been reported to participate in diverse cellular processes. It was found that the amino-acid residues from R228 to F237 function as a unique nuclear translocation signal (NTS) and are required for nuclear translocation of ANXA1.
Transactivator of transcription (Tat) is a cell-penetrating peptide. With high efficiency, the peptide can translocate numerous proteins, peptides, DNA, RNA, and small drugs into the cytoplasm. The Tat-NTS (NH2-YGRKKRRQRRR-RSFPHLRRVF-CONH2) peptide was found to block the interaction of ANXA1 with importing, specifically β. This blocking would inhibit the nuclear translocation of ANXA1, consequently protecting neurons from ischemic stroke damage. So, the Tat-NTS peptide can be used as a novel and potentially promising new therapeutic candidate for the treatment of ischemic stroke.
From the experiment, the Tat-NTS peptide-treated mice displayed remarkable cognitive improvement. In addition, the Tat-NTS peptide had no noticeable effect on neuronal apoptosis. So the administration of Tat-NTS peptide to dissociation of ANXA1–importin β interaction may be a new drug in ischemic stroke therapy.
The Tat sequence is originally from a crucial non-structural protein of human immunodeficiency virus (HIV). The function of Tat is to bind to the viral long terminal repeat (LTR) and activate cellular transcription machinery to initiate transcription of the viral proteins. Later, this 11-amino acid positively charged peptide was found to pull diverse molecules across cell membranes in vitro and in vivo. Fusion proteins constructed with TAT rapidly enter and exit cells and cross intracellular membranes. Electrostatic interactions between TAT and the cell membrane have been implicated as a part of the transduction mechanism. Neither apoptosis nor necrosis is induced in cells after exposure to TAT.
https://www.nature.com/articles/s41418-018-0116-5