Fluorescent peptide labeling has become an indispensable tool for studying biomolecular interactions, cellular dynamics, and therapeutic development. Among the fluorophores widely adopted for this purpose, TAMRA (Tetramethylrhodamine) is a standout choice due to its bright emission, photostability, and versatility in peptide conjugation. This article examines the methodologies, advantages, and scientific applications of TAMRA-based fluorescent peptide labeling, emphasizing its critical role in biochemical and biomedical research.
Key Takeaways
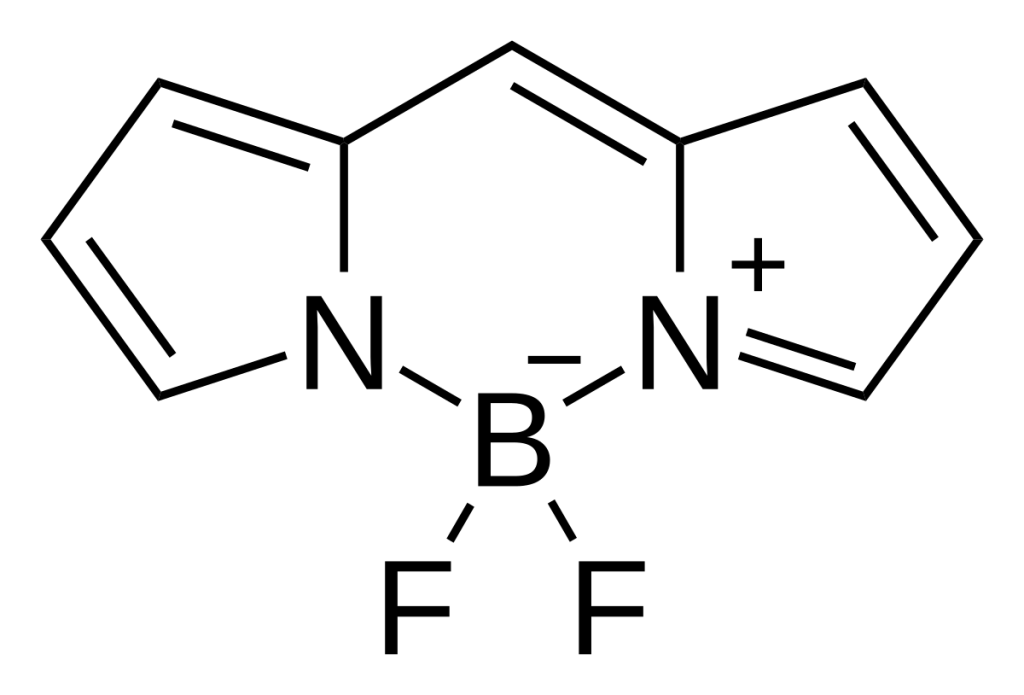
- TAMRA is a rhodamine-derived dye with excitation and emission peaks at 555 nm and 580 nm, optimized for red-channel fluorescence detection.
- It is frequently used for peptide labeling via NHS ester chemistry, enabling covalent bonds with primary amines (e.g., lysine residues or peptide N-termini).
- TAMRA-labeled peptides are essential for live-cell imaging, flow cytometry, and fluorescence resonance energy transfer (FRET) experiments.
- The dye’s pH sensitivity and hydrophobicity necessitate careful optimization during labeling protocols.
- Lifetein provides custom TAMRA-labeled peptide synthesis, ensuring high-purity conjugates for diverse research needs.
Introduction to TAMRA: A Rhodamine-Based Fluorophore
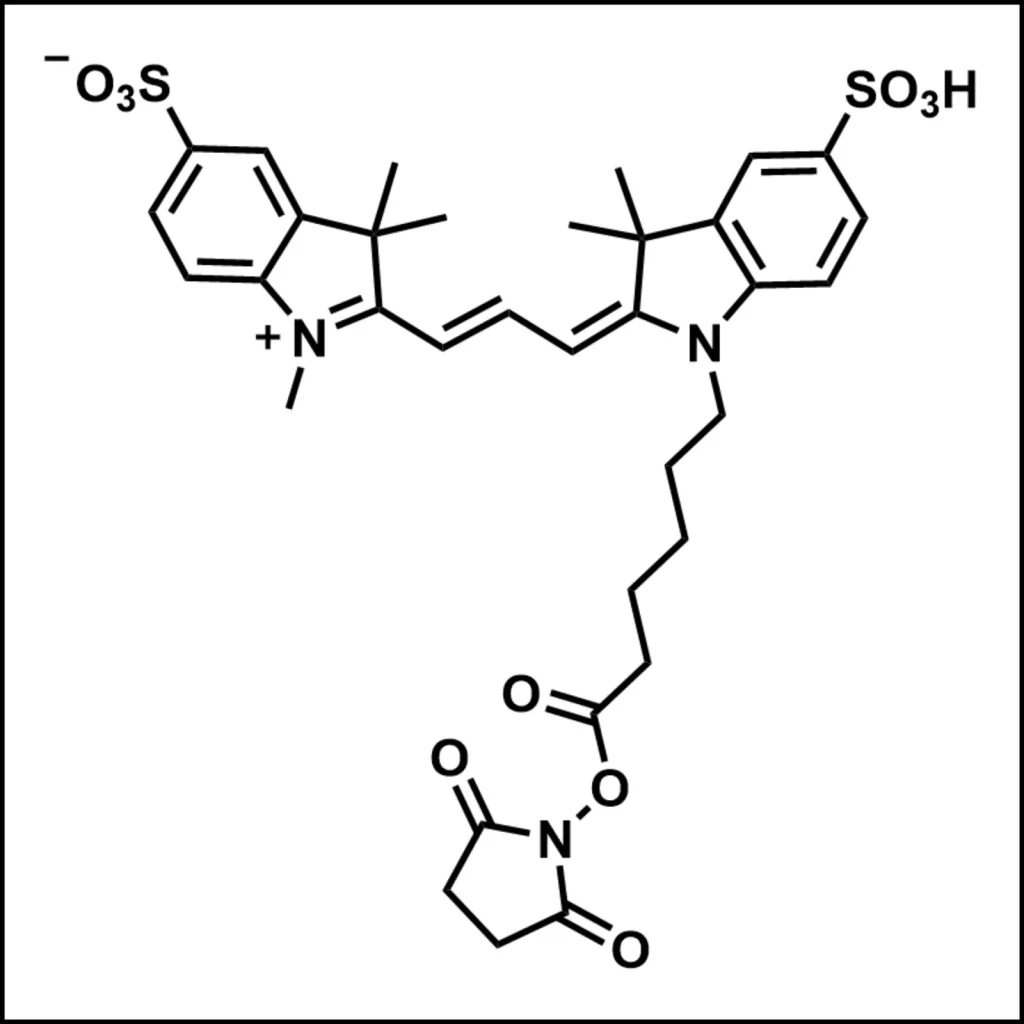
TAMRA, a member of the rhodamine dye family, is renowned for its bright orange-red fluorescence and robust photophysical properties. With a molar extinction coefficient of approximately 90,000 M⁻¹cm⁻¹ and a quantum yield of 0.3–0.5, it generates strong signals in fluorescence microscopy and spectroscopy. Unlike cyanine dyes such as Cy3, TAMRA exhibits pH-dependent fluorescence, performing optimally in neutral to slightly acidic environments. This characteristic requires meticulous buffer selection during experimental design.
Structurally, TAMRA contains reactive groups such as NHS esters or maleimides, which facilitate covalent conjugation to peptides. Its compatibility with solid-phase peptide synthesis (SPPS) allows for site-specific labeling, preserving peptide functionality and minimizing structural disruption.
Applications of TAMRA-Labeled Peptides
Live-Cell Imaging and Subcellular Tracking
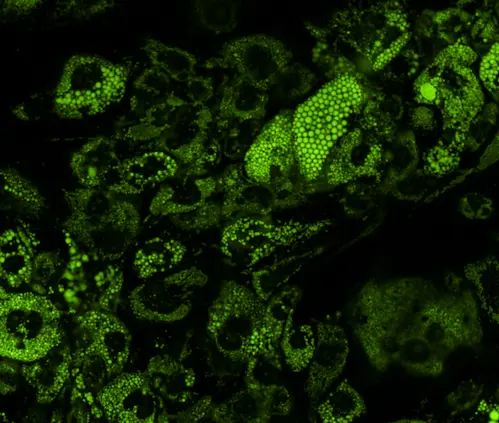
TAMRA-labeled peptides are widely employed to monitor cellular uptake, subcellular localization, and real-time trafficking in live cells. For example, TAMRA-conjugated cell-penetrating peptides (CPPs) enable visualization of intracellular delivery mechanisms. The dye’s photostability ensures minimal signal loss during prolonged imaging sessions, a critical feature for time-lapse microscopy.
Protein-Protein Interaction Studies
In FRET-based assays, TAMRA acts as an acceptor dye paired with donors like fluorescein. This configuration allows detection of molecular interactions between labeled peptides and target proteins. For instance, TAMRA-labeled kinase substrate peptides can reveal enzymatic activity by quantifying changes in FRET efficiency upon phosphorylation.
Diagnostic and Therapeutic Development
TAMRA’s bright emission makes it valuable in diagnostic probes and drug delivery systems. Peptides labeled with TAMRA and designed to target biomarkers (e.g., tumor-specific receptors) are used in fluorescence-guided surgery or as components of theranostic nanoparticles.

Methodologies for TAMRA Peptide Labeling
NHS Ester Chemistry
The most common labeling method involves conjugating TAMRA NHS esters to primary amines on peptides. This reaction occurs under mild alkaline conditions (pH 8.0–9.0) in amine-free buffers such as PBS or sodium bicarbonate. Key considerations include:
- Peptide Solubility: TAMRA’s hydrophobicity may reduce solubility, necessitating organic solvents (e.g., DMSO) or detergents.
- Degree of Labeling (DOL): Excessive labeling (>1 dye per 10 amino acids) risks fluorescence quenching or peptide aggregation.
Site-Specific Labeling via SPPS
During solid-phase peptide synthesis, TAMRA can be incorporated at specific residues (e.g., lysine or cysteine) using orthogonal protecting groups. Lifetein specializes in this approach, offering custom-labeled peptides with precise dye placement. Post-synthesis purification via reverse-phase HPLC removes unreacted dye and impurities.
Maleimide-Based Thiol Conjugation
For cysteine-containing peptides, TAMRA maleimide offers a thiol-specific labeling option. This method is ideal for peptides lacking lysine residues or requiring N-terminal modifications.
Challenges and Optimization Strategies
pH Sensitivity
TAMRA’s fluorescence intensity diminishes in alkaline environments (pH >8.0). To address this, researchers use pH-stabilized buffers (e.g., HEPES) or maintain controlled pH conditions during imaging.
Solubility and Aggregation
TAMRA’s hydrophobic nature can lead to peptide aggregation. Mitigation strategies include:
- Adding polar linkers (e.g., PEG spacers) between the dye and peptide.
- Using lyophilization-resistant formulations during synthesis.
Signal-to-Noise Ratio
Background fluorescence from unbound dye or cellular autofluorescence can obscure signals. Rigorous HPLC purification and blocking steps (e.g., BSA in staining buffers) enhance signal clarity.
FAQ
What distinguishes TAMRA from other fluorescent dyes like Cy3 or FITC?
TAMRA is a rhodamine-derived dye with excitation/emission maxima at 555 nm/580 nm, making it ideal for red-channel detection. Unlike FITC (which emits in the green spectrum) or Cy3 (a cyanine dye with orange emission), TAMRA offers superior photostability and pH-dependent fluorescence. However, it is more hydrophobic than Cy3, which can complicate solubility.
When should I use NHS ester vs. maleimide chemistry for TAMRA labeling?
- NHS ester chemistry targets primary amines (lysine residues or N-termini) and is ideal for peptides with accessible amine groups.
- Maleimide chemistry reacts with thiol groups (cysteine residues), making it suitable for peptides lacking lysine or requiring site-specific labeling.
Choose based on peptide sequence and functional group availability.
Why does TAMRA fluorescence diminish at high pH?
TAMRA’s fluorescence is pH-sensitive due to its rhodamine backbone, which undergoes structural changes in alkaline environments. At pH >8.0, the dye’s zwitterionic form shifts, reducing quantum yield. Use pH-stabilized buffers (e.g., HEPES) or maintain neutral conditions during experiments.