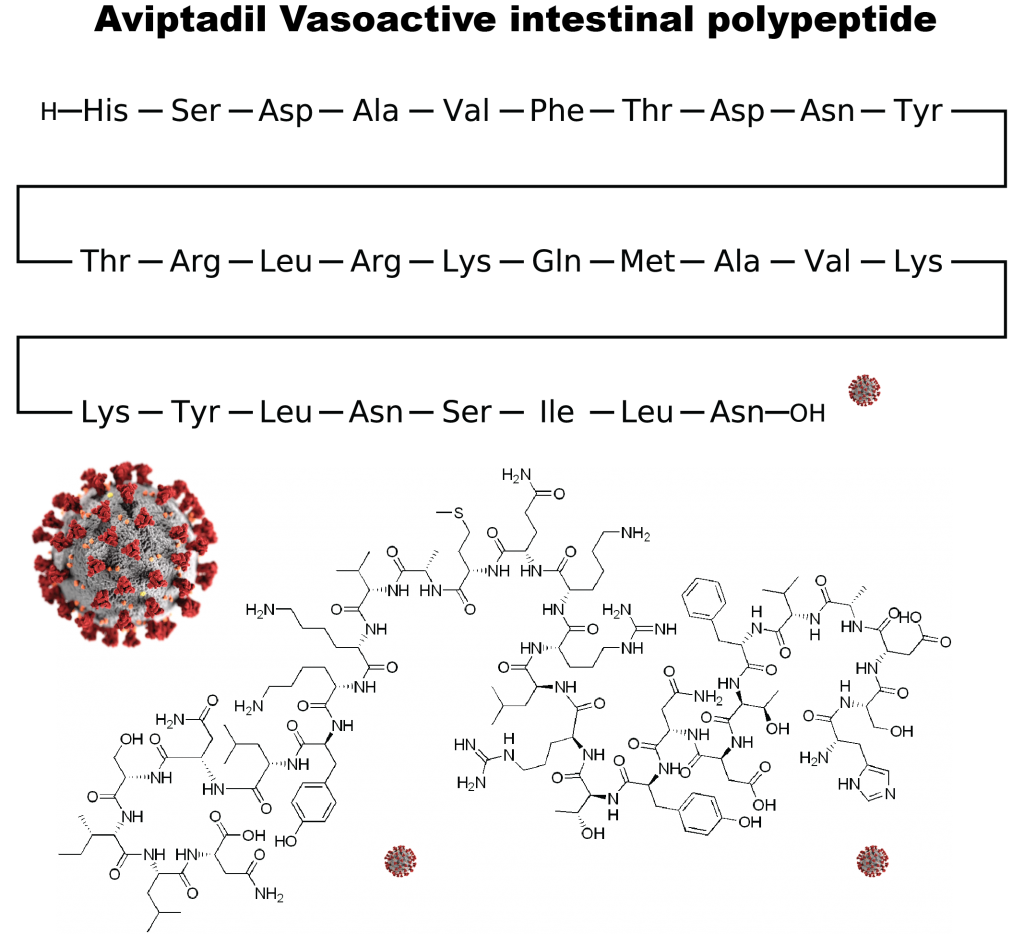
It was found that RLF-100 (Aviptadil) is associated with rapid respiratory failure recovery among COVID-19 Patients. The clinical findings may be based on evidence that VIP inhibits the replication of the SARS-CoV-2 virus in human lung cells and immune cells (monocytes). No other antiviral agent has demonstrated rapid recovery from viral infection and demonstrated laboratory inhibition of viral replication. It is a patented formulation of aviptadil (synthetic human Vasoactive Intestinal Polypeptide, VIP), which has been granted FDA fast track designation, FDA emergency use IND authorization, and an expanded access protocol. Aviptadil is an injectable formulation of the vasoactive intestinal polypeptide (VIP) in combination with the adrenergic drug phentolamine.