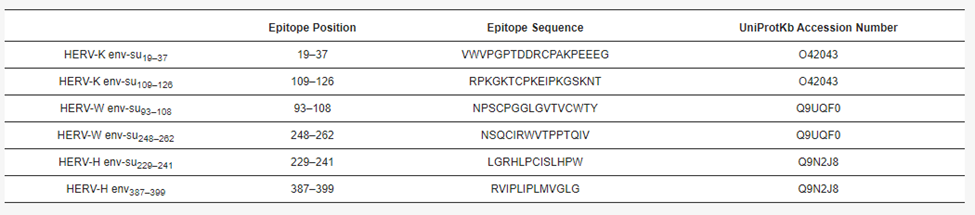
Expression of human endogenous retroviruses (HERVs) shows potential for peptide derivatives to be used as biomarkers for prostate cancer. Specifically, peptides from HERV-K and HERV-H Proteins show association in prostate cancer pathogenesis.
HERV Peptides as Biomarkers for Prostate Cancer
With prostate cancer being the most common cause of death by cancer in males, there is a need to identify aggressive tumors that current diagnostic tests do not measure up to. Scientists looked toward the envelope protein of HERV family viruses, well known for its immunosuppressive properties and role in modulating transcription factors of cancer-associated pathways. LifeTein synthesized the peptide derivatives of this protein, where HERV-K and HERV-H especially showed promise as prostate cancer biomarkers.
The findings suggest these HERV peptides have capabilities for their serum autoantibodies to further investigate the expression levels of the envelope protein of HERV-K and HERV-H in biopsy samples. It remains ever exciting to watch the continuously-growing usefulness of peptides expand into more and more fields, and hopefully use as Biomarkers is far from the last.
Manca, M.A.; Solinas, T.; Simula, E.R.; Noli, M.; Ruberto, S.; Madonia, M.; Sechi, L.A. HERV-K and HERV-H Env Proteins Induce a Humoral Response in Prostate Cancer Patients. Pathogens 2022, 11, 95. https://doi.org/10.3390/pathogens11010095