Fluorescent peptide labelling with Cy5, a cyanine dye, has become an indispensable technique in biomedical research, enabling the precise visualization and tracking of peptides in complex biological systems. This method leverages the exceptional photophysical properties of Cy5, which emits in the red to near-infrared region (approximately 650 nm excitation and 670 nm emission), to facilitate a wide range of applications from live-cell imaging to molecular interaction studies. The high molar extinction coefficient and structural versatility of Cyanine5 make it particularly valuable for experiments requiring deep tissue penetration and minimal background autofluorescence, thereby providing enhanced sensitivity and specificity. Consequently, the strategic implementation of Cy5 labelling allows researchers to monitor peptide internalization, investigate protein-protein interactions, and develop advanced diagnostic assays with remarkable clarity and precision.
Key Takeaways
- Cy5 is characterized by its high molar extinction coefficient and fluorescence in the red to near-infrared spectrum (Ex ~650 nm, Em ~670 nm), which minimizes background interference and is ideal for deep tissue imaging.
- Common applications include live-cell imaging, receptor internalization studies, FRET-based assays, and the development of sensitive biosensors for pathogen detection.
- Labelling can be achieved through site-specific methods such as maleimide-thiol coupling or click chemistry, often utilizing a C-terminal cysteine for controlled conjugation.
- While relatively stable, considerations such as potential photobleaching and the need for efficient purification post-labelling are crucial for maintaining signal integrity and quantitative accuracy.
- Commercial providers like LifeTein offer comprehensive services for synthesizing labeled peptides, supporting research with a wide array of fluorescent dye options.
Introduction to Cy5 and Its Photophysical Properties
Chemical Characteristics of Cyanine Dyes
Cyanine dyes, including Cy5, belong to a class of synthetic fluorescent molecules characterized by a polymethine bridge connecting two nitrogen-containing aromatic rings. This structure confers a delocalized positive charge, contributing to high extinction coefficients and tunable absorption and emission profiles based on the chain length and chromophores. Cy5, specifically, is a fat-soluble dye that can be modified with sulfonic acid groups to create water-soluble derivatives, enhancing its compatibility with biological systems without significantly altering its optical properties. The dye’s substantial size, however, means it can potentially perturb the biological activity of the labeled peptide, necessitating careful functional validation after conjugation.
Spectral Advantages for Bioimaging
The primary advantage of Cy5 lies in its fluorescence emission in the near-infrared window, which ranges from approximately 650 nm to 670 nm. This spectral range is associated with reduced light scattering and minimal absorption by hemoglobin and water in biological tissues, thereby allowing for deeper penetration and lower background autofluorescence compared to visible light-emitting fluorophores. Consequently, the dye is exceptionally suited for in vivo imaging applications. Furthermore, its compatibility with standard filters for flow cytometry and fluorescence microscopy makes it a versatile choice for various detection platforms.
Find out more about fluorescent peptides here.
Applications of Cy5-Labelled Peptides in Research
Fluorescence Resonance Energy Transfer (FRET)
Cy5 is frequently employed as an acceptor dye in FRET pairs, where it interacts with a donor fluorophore such as Cy3. This configuration is utilized to study protease activity, protein-protein interactions, and conformational changes in peptides. The efficiency of energy transfer in FRET is highly dependent on the proximity between the donor and acceptor, making Cy5-labeled peptides ideal for monitoring molecular interactions and enzymatic cleavage events in real-time. Standardized FRET pairs incorporating the dye are widely available and supported by commercial peptide synthesis services.
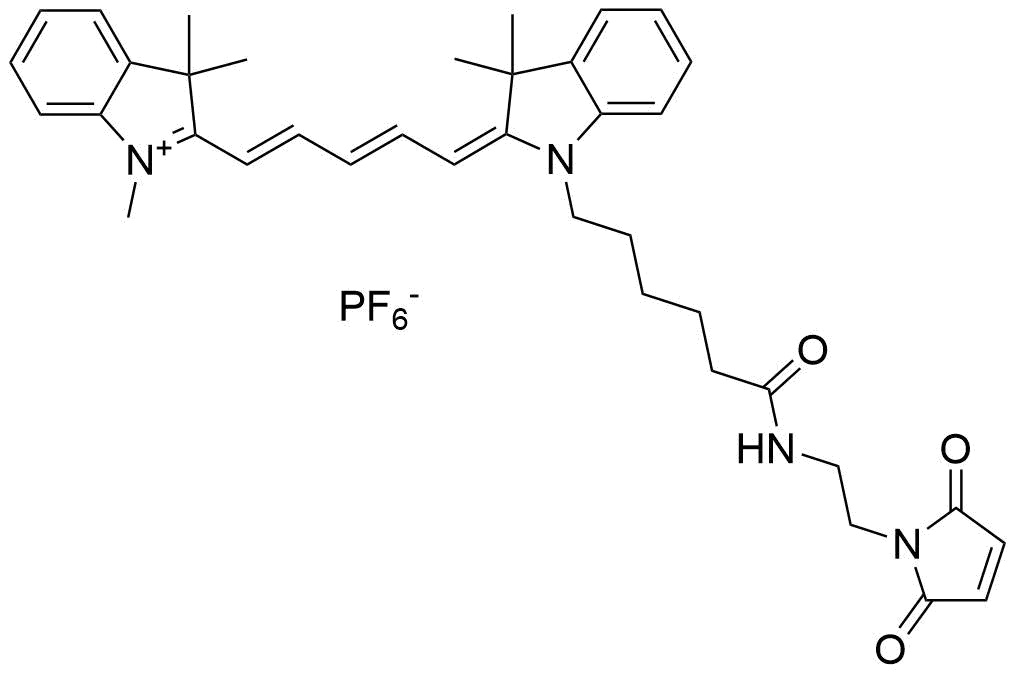
Conjugation Strategies and Practical Considerations
Site-Specific Labelling Techniques
Achieving site-specific conjugation of Cy5 to peptides is essential for preserving biological activity and ensuring reproducible results. Common strategies include:
- Maleimide-thiol chemistry: This method targets cysteine residues, typically introduced at the C-terminus or specific positions within the peptide sequence. The reaction between the maleimide-functionalized Cy5 and the thiol group of cysteine is highly efficient and selective, allowing for controlled labeling with minimal side products.
- Click chemistry: Copper-catalyzed azide-alkyne cycloaddition (CuAAC) is another robust approach, where an azide-containing peptide reacts with an alkyne-functionalized Cy5 dye. This method offers excellent specificity, compatibility with aqueous buffers, and the ability to label peptides in complex mixtures.
Additionally, conjugation to primary amines (e.g., on lysine residues or the N-terminus) using NHS ester derivatives of Cy5 is a conventional method, though it may result in heterogeneous labeling if multiple amines are present.
Purification and Validation
Following the conjugation reaction, high-performance liquid chromatography (HPLC) is typically employed to purify the Cy5-labeled peptide from unreacted dye and impurities. Subsequent validation using mass spectrometry (MS) confirms the identity and molecular weight of the conjugate, ensuring labeling efficiency and product correctness. It is also critical to perform functional assays to verify that the Cy5 modification does not adversely affect the peptide’s binding affinity or biological activity, as demonstrated in cAMP accumulation studies for GPCR-targeted peptides.
Find out more about peptide synthesis here.
Frequently Asked Questions (FAQ)
What are the excitation and emission maxima of Cy5?
Cy5 exhibits peak excitation at approximately 650 nm and emission at approximately 670 nm, placing it in the red to near-infrared region of the spectrum. This makes it well-suited for applications requiring minimal background interference and deep tissue penetration.
Can Cy5 be used for in vivo imaging?
Yes, the near-infrared emission properties of the dye make it an excellent fluorophore for in vivo imaging. It allows for non-invasive visualization of biological processes in live animals, such as tumor targeting and biodistribution studies, with high contrast due to reduced absorption by tissue components.
How is Cy5 specifically conjugated to peptides?
Cy5 is typically conjugated using site-specific methods such as maleimide-thiol chemistry (targeting cysteine residues) or click chemistry (via azide-alkyne cycloaddition). These approaches ensure controlled labeling at defined positions, which is crucial for maintaining the peptide’s functional integrity.
