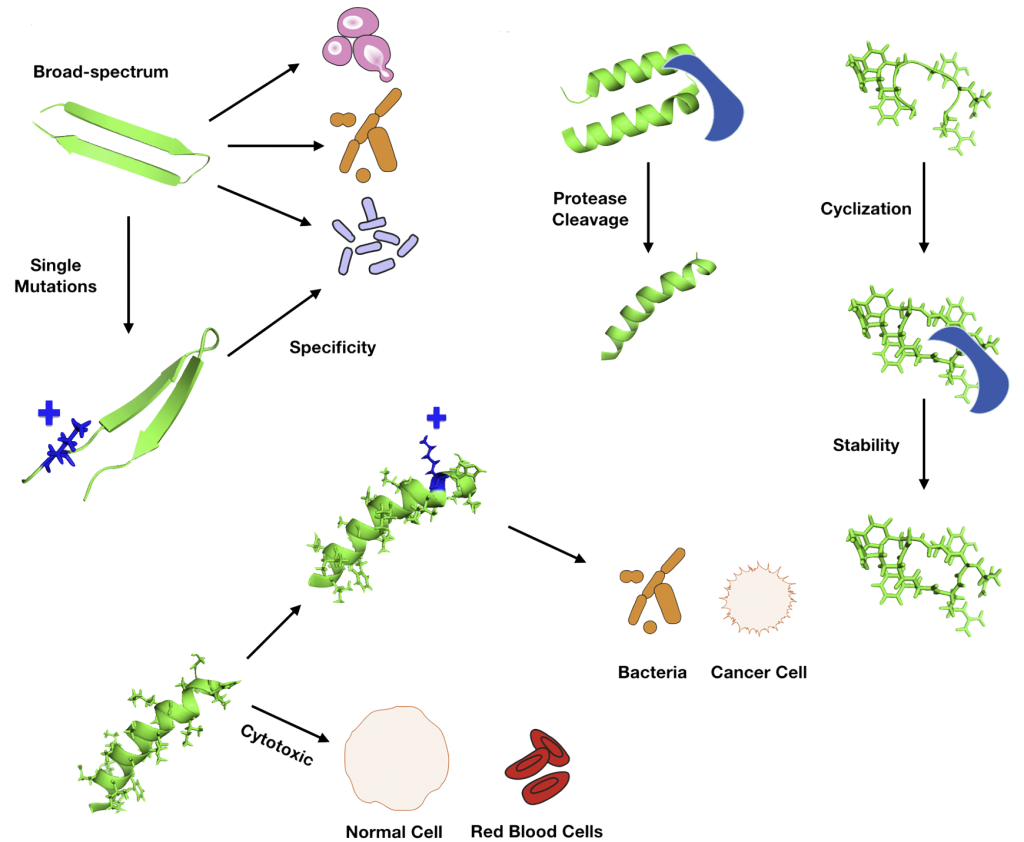
Antimicrobial peptides (AMPs) represent promising alternatives to conventional antibiotics. These antimicrobial peptides form pores by interaction with the peptide and lipid from bacterial membranes. Thus, AMPs will interfere with cellular processes and metabolic pathways.
Peptidomimetics are to mimic natural peptides three-dimensionally in order to preserve their biological activity and obtain high stability, resistance to proteolysis, and bioavailability. Slight modifications in the amino acid composition can change the geometrical disposition and physicochemical properties of a peptide.
The most common features relevant for AMP design are net charge, hydrophobicity, and helix penalty of each residue separately.
Natural AMPs vary from +3 to +6 net charge because the bacterial membranes have large quantities of negatively charged molecules. The bacterial membrane is composed mostly of lipids. Therefore, hydrophobicity is an important feature for designing peptides with antimicrobial activity. The percentage of hydrophobic residues in naturally occurring peptides are between 40% and 60%.
Modifying amino acids can enhance the stability of peptides by increasing their resistance to proteolytic degradation. An example of this approach is to replace tryptophan or histidine residues with the bulky amino acid beta-naphthylalanine.
Peptides are promising compounds not only for antimicrobial therapeutics but as immunomodulatory agents and anticancer drugs.
