Glycosylation is the most abundant polypeptide chain modification in nature. Glycoproteins are a significant class of current therapeutic targets and clinical biomarkers.
Glycans can be covalently attached to the amide nitrogen of Asn residues (N-glycosylation), to the hydroxyl oxygen of Ser or Thr residues (O-glycosylation), and the indole C2 carbon of Trp through a C–C linkage (C-mannosylation).
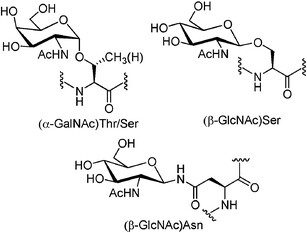
Glycosylation of peptides is a promising strategy for modulating the physicochemical properties of peptide drugs and for improving their absorption through biological membranes. The glycosylated peptide can target specific organs, enhance the biodistribution in tissues, improve penetration through biological membranes, increase metabolic stability and lower the clearance rate, receptor-binding, protect amino acid’s side chain from oxidation, and maintain and stabilize the physical properties of peptides, such as precipitation, aggregation and thermal and kinetic denaturation.
LifeTein provides the synthesis of glycoconjugates and the development of glycosylated peptide therapeutics. We have developed the glycosylated amino acids, which are compatible with standard protocols in Fmoc solid phase peptide synthesis. The pre-synthesized glycosylated amino acid is coupled to the elongating peptide using solid phase peptide synthesis (SPPS) in a stepwise fashion. Our typical synthesis is for the peptide of 10-20 amino acids. The integration of long peptides with more than 50 residues is difficult by stepwise synthesis, due to the incomplete couplings and epimerization.

The modification of peptides with different sugar entities, such as mannose, galactose, and N-acetylgalactosamine (GalNAc), exerts diverse impacts on the conformational properties of the polypeptide chain. The position of the glycosyl unit in the peptide’s structure is an essential factor in changing the conformation of the peptide backbone and may affect the biological properties of the modified peptide. LifeTein is willing to work with you on the development of therapeutic peptides using different glycosylation strategies. For example, the improved permeability and higher metabolic stability of the glycosylated neuropeptides resulted in a significant increase in their bioavailability, which might account for the enhanced analgesic effect of the glycopeptides.
LifeTein’s technology will help your development of carbohydrate-modified peptide drugs.
| Name | CAS | Formula |
| Fmoc-L-Ser((Ac)3-β-D-GlcNAc)-OH | 160067-63-0 | C32H36N2O13 |
| Fmoc-L-Thr((Ac)3-β-D-GlcNAc)-OH | 160168-40-1 | C33H38N2O13 |
| FMoc-Asn(β-D-GlcNAc(Ac)3)-OH | 131287-39-3 | C33H37N3O13 |
| beta-D-Glucose pentaacetate | 604-69-3 | C16H22O11 |
| Gluconic acid | 526-95-4 | C6H12O7 |
| 6- | 921-62-0 | C6H13O10P |
| 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl isothiocyanate | 14152-97-7 | C15H19NO9S |
| Name | CAS | Formula |
| Fmoc-L-Ser((Ac)3-β-D-GalNAc)-OH | 1676104-71-4 | C32H36N2O13 |
| Fmoc-L-Ser((Ac)3-α-D-GalNAc)-OH | 120173-57-1 | C32H36N2O13 |
| Fmoc-Thr(GalNAc(Ac)3-α-D)-OH | 116783-35-8 | C33H38N2O13 |
| Fmoc-L-Thr(β-D-GalNAc(Ac)3)-OH | 133575-43-6 | C33H38N2O13 |
| beta-D-Galactose pentaacetate | 4163-60-4 | C16H22O11 |
| 1,2,3,4,6-Penta-O-acetyl-α-D-galactopyranose | 4163-59-1 | C16H22O11 |
| Name | CAS | Formula |
| Fmoc-L-Ser(ManNAc)-OH | ||
| Fmoc-Thr(ManNAc)-OH | ||
| α-D-MANNOSE PENTAACETATE | 4163-65-9 | C16H22O11 |
| D-MANNOSE PENTAACETATE | 25941-03-1 | C16H22O11 |
| D-Mannopyranose tetraacetate | 140147-37-1 | C14H20O10 |
