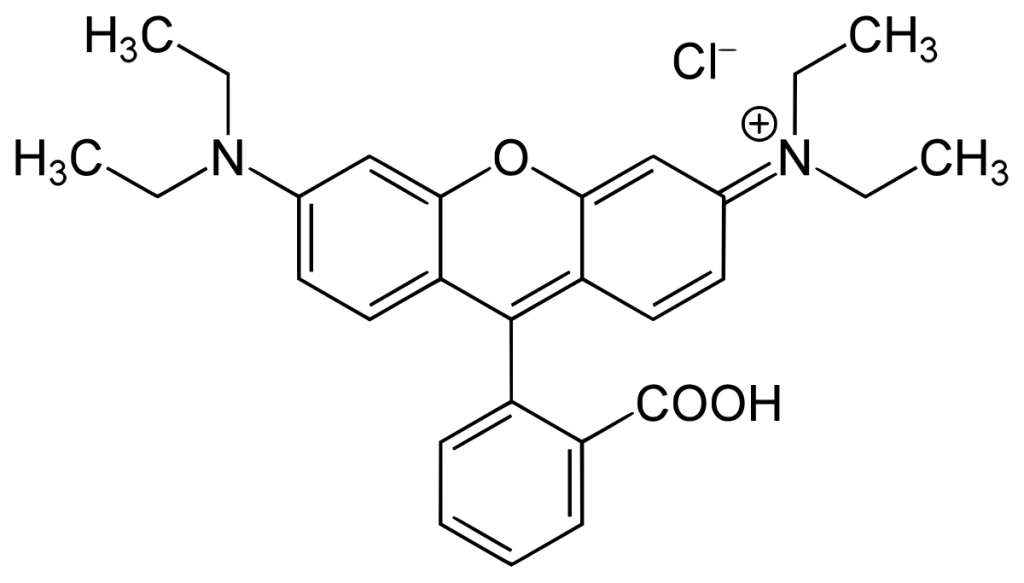
Rhodamine B is a xanthene-derived fluorescent dye renowned for its high quantum yield and photostability, making it invaluable across biological imaging, diagnostic assays, and biomolecular tracking. This dye exhibits optimal excitation and emission wavelengths near 570 nm and 590 nm, respectively, positioning it within the orange-red spectrum ideal for minimizing background autofluorescence in biological samples. Its chemical structure, featuring a hydrophilic carboxyl group and hydrophobic diethylamino groups, allows versatile conjugation to peptides, proteins, and other biomolecules via isothiocyanate (NHS ester) chemistry, primarily targeting primary amines. Nevertheless, environmental factors such as pH, solvent polarity, and interactions with biomolecules can significantly influence its fluorescence properties, necess careful experimental design. As a cornerstone in fluorescence microscopy, flow cytometry, and FRET-based assays, Rhodamine B continues to evolve through synthetic refinements that enhance its brightness, stability, and applicability in advanced research contexts.
Key Takeaways
- Rhodamine B exhibits excitation/emission maxima at ~570/590 nm, ideal for red-shifted fluorescence applications.
- Conjugation primarily targets primary amine groups via isothiocyanate or NHS ester chemistry, often incorporating spacers like aminohexanoic acid (Ahx) to reduce steric hindrance.
- Fluorescence intensity and lifetime are influenced by microenvironmental factors (e.g., pH, viscosity, and protein interactions).
- Applications span live-cell imaging, FRET probes, biosensing, and glycan analysis due to its high molar extinction coefficient and quantum yield.
Chemical Properties and Spectral Characteristics
Structural Basis of Fluorescence
Rhodamine B’s fluorescence stems from its rigid xanthene core and electron-donating diethylamino groups, which create a conjugated system enabling efficient light absorption and emission. The dye exists in a pH-dependent equilibrium between a fluorescent zwitterionic form and a non-fluorescent lactone form, with the zwitterion dominating in aqueous solutions at neutral pH. This balance is critical for its performance, as extreme pH or non-polar environments can shift equilibrium toward the non-emissive state, quenching fluorescence.
Spectral Profiles and Environmental Sensitivity
The dye’s excitation and emission maxima (~570/590 nm) make it particularly suitable for applications requiring separation from endogenous fluorophores like chlorophyll or hemoglobin. However, its fluorescence lifetime and quantum yield are highly sensitive to local viscosity, temperature, and specific interactions with biomolecules. For instance, binding to proteins can enhance fluorescence by restricting molecular motion, while alkaline conditions may promote lactonization, reducing signal output.
Find out more about fluorescent peptides here.
Conjugation Strategies and Techniques
Covalent Attachment of Rhodamine B to Biomolecules
Rhodamine B is typically conjugated to target molecules via its reactive isothiocyanate (-NCS) or N-hydroxysuccinimide (NHS ester) derivatives, which form stable thiourea or amide bonds with primary amines (e.g., lysine residues or N-termini of peptides). To mitigate steric hindrance, especially in densely labeled proteins or peptides, spacers like aminohexanoic acid (Ahx) are incorporated, improving labeling efficiency and preserving biological activity.
Optimization of Labeling Conditions
Successful conjugation requires precise control of pH (typically pH 8-9) to ensure amine group reactivity while avoiding dye precipitation. Post-labeling purification via HPLC or gel filtration is essential to remove unreacted dye, which could contribute to background noise. LifeTein’s protocols, for example, emphasize orthogonal protection strategies and stepwise purification to achieve high-precision labeled products.
Biological and Analytical Applications
Live-Cell Imaging and Trafficking
Rhodamine B’s photostability and cell permeability make it a preferred tag for tracking peptide internalization, protein localization, and organelle dynamics. Notably, its derivatives enable lysosome-specific staining due to pH-dependent activation in acidic environments (pH 4.5-5.0) 5. Furthermore, Rhodamine B-labeled cell-penetrating peptides (CPPs) facilitate real-time monitoring of cytosolic delivery, as demonstrated in studies involving Tat and penetratin conjugates.
FRET-Based Protease Sensing
In FRET assays, Rhodamine B serves as an acceptor paired with donors like fluorescein or cyanine dyes. For example, peptides labeled with Rhodamine B and a quencher (e.g., Dabcyl) exhibit minimal fluorescence until protease cleavage separates the pair, generating a detectable signal. This principle underpins assays for HIV protease, caspase, and other enzymatic activities, offering high sensitivity for drug screening and mechanistic studies.
Find out more about peptide synthesis here.

Challenges and Practical Considerations
Synthetic and Handling Complexities of Rhodamine B
Synthesis of Rhodamine B conjugates requires anhydrous conditions to prevent hydrolysis of reactive esters. Additionally, the dye’s tendency to form aggregates in aqueous buffers may necessitate co-solvents (e.g., DMSO) for solubilization. LifeTein’s workflows address these issues through optimized coupling protocols and quality controls (e.g., MS/HPLC verification).
Frequently Asked Questions (FAQ)
How does pH affect Rhodamine B fluorescence?
Rhodamine B is generally pH-insensitive between pH 4-9 but may undergo lactonization under strongly alkaline conditions (pH >10), quenching fluorescence. Conversely, extreme acidity (pH <2) can protonate the diethylamino groups, reducing quantum yield.
Can Rhodamine B be used for dual-labeling with other fluorophores?
Yes, its emission spectrum overlaps minimally with common blue/green dyes (e.g., FITC, Alexa 488), enabling multiplexed imaging. However, spectral cross-talk should be assessed via control experiments.
What is the typical degree of labeling (DOL) achievable for proteins?
DOL depends on target accessibility but usually ranges from 1-3 dye molecules per protein. Higher DOL may cause self-quenching or functional impairment.
How stable are Rhodamine B conjugates during storage?
Conjugates are stable at -20°C for months when protected from light. Lyophilization is recommended for long-term storage, albeit with potential aggregation risks upon reconstitution.
Feng, Y., Liu, W., Mercadé-Prieto, R., & Chen, X. D. (2021). Dye-protein interactions between Rhodamine B and whey proteins that affect the photoproperties of the dye. Journal of Photochemistry and Photobiology A: Chemistry, 408, 113092. https://doi.org/10.1016/j.jphotochem.2020.113092
Zhang, X.-F., Zhang, Y., & Liu, L. (2014). Fluorescence lifetimes and quantum yields of ten rhodamine derivatives: Structural effect on emission mechanism in different solvents. Journal of Luminescence, 145, 448–453. https://doi.org/10.1016/j.jlumin.2013.07.066
Dusa, F., Smolkova, D., Cmelik, R., Guttman, A., & Lavicka, J. (2025). Labeling of oligosaccharides and N-linked glycans by a rhodamine-based fluorescent tag for analysis by capillary electrophoresis with laser-induced fluorescence and mass spectrometry detection. Talanta, 286, 127456. https://doi.org/10.1016/j.talanta.2024.127456
