Some fusion or chimeric proteins could never be produced from the E.coli expression system, especially when several hydrophobic sequences are involved in the functional domains. Obtaining peptides sized 100–200 amino acids using chemical synthesis is much faster and cheaper than cloning and overexpressing in Escherichia coli. In addition, the resulting peptide is always correct. Chemical synthesis can regularly incorporate non-genetically encoded structures, such as D-amino acids, into the protein. Synthetic peptides eliminate problems such as poor or no expression, cloning errors, tags like FLAG or 6-His, or the mistranslation of non-preferred codons in prokaryotic hosts. Artificial amino acids that have isosteric side chains can be used to investigate the functional importance of specific residues. The peptide design and synthesis using click chemistry can achieve all these chimeric proteins.
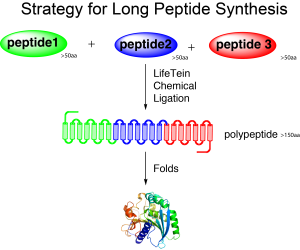
Long peptide synthesis by click chemistry
