Various modifications have been incorporated in the peptides such as acetylation, amidation, cyclization, PEGylation, glycosylation, succinylation, and hydroxylation, to increase the half-life of peptides.
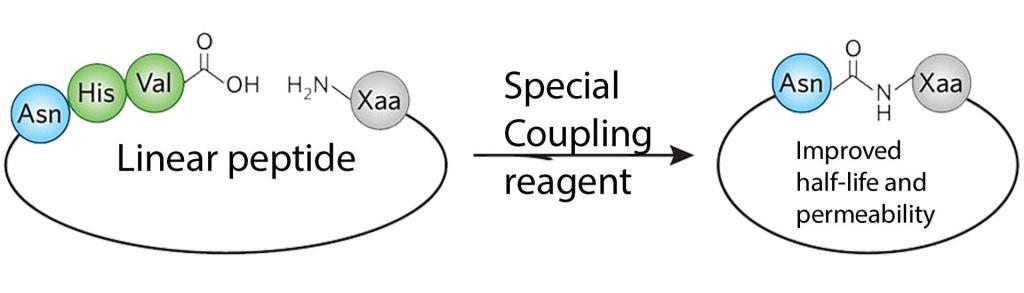
Bioactive head-to-tail cyclic peptides are promising lead structures for the development of new pharmaceuticals with their high selectivity, potency, and improved enzymatic stability.
Cyclic peptides are usually synthesized with an N-terminal amide linkage that closes the ring structure with a C-terminal carboxylic acid. The current methods for amide formation are expensive and inelegant as the top challenge for organic chemistry. The issues of waste and expense associated with amide formation are responsible for the enormous cost of commercial therapeutic peptides. The reaction typically renders a very low yield.
Recently, LifeTein developed a single-step preparation of the amide cyclization with a high yield. The resulting peptide macrocycles are conformationally stable with multiple intramolecular hydrogen bonds. LifeTein’s advances in amide-forming methodologies can have far-reaching impacts across scientific disciplines. The unnatural amino acids can be easily incorporated during the synthesis for stability or enhanced activity, or specific probes for interrogating binding and biological function during the synthesis. The improved amide-forming chemical reactions will not damage the structure of any unnatural amino acids.
This cyclization method allows us to synthesize complex, highly functionalized amide-based structures without the need for aggressive reactants, expensive protecting groups and longer reaction times.
