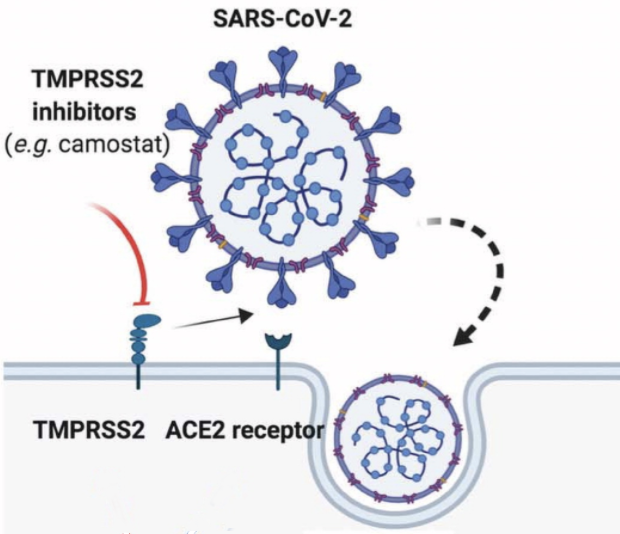
Transmembrane serine protease 2 (TMPRSS2) is a serine protease that in humans is encoded by the TMPRSS2 gene. It is a cell surface protein primarily expressed by endothelial cells across the respiratory and digestive tracts. The protein contains a type II transmembrane domain, a receptor class A domain, a scavenger receptor cysteine-rich domain and a protease domain.
Recent evidence suggested that SARS-CoV-2 uses the ACE2 receptor for cell entry, in synergy with the host’s TMPRSS2. The viral S glycoprotein is cleaved by TMPRSS2, thus facilitating viral activation. As TMPRSS2 is a serine protease, it primes the spike-domain (S) of SARS-CoV-2 by cleaving as the S1/S2 sites. TMPRSS2 activity is crucial for cell entry and viral pathogenesis. In a recent in vitro study by Hoffmann et al., the TMPRSS2 inhibitor camostat mesylate blocked the SARS-CoV-2 entry into primary lung cells, suggesting that TMPRSS2 could represent a potential target in SARS-CoV-2 treatment. This drug is approved for clinical use already in Japan for unrelated illnesses and could serve to be an important therapy for COVID-19.