
Tryptophan is an essential amino acid, meaning the human body cannot synthesize it and must obtain it through diet. It serves as a critical building block for proteins and a precursor to key molecules, most notably the neurotransmitter serotonin and the hormone melatonin. This biochemical relationship to sleep-regulating compounds is the foundation of tryptophan’s popular reputation as a drowsiness-inducing agent, particularly in the context of holiday turkey consumption. However, the biological reality of how tryptophan affects sleep is more complex and nuanced than the common myth suggests. Furthermore, beyond its role in nutrition, tryptophan’s unique chemical structure makes it a valuable and versatile tool in the field of peptide synthesis and pharmaceutical development.
Key Takeaways
- The amount of tryptophan in a typical serving of turkey is not exceptional compared to other common poultry and meats, challenging the basis of the “Thanksgiving sleepiness” myth.
- Isolated, high-dose tryptophan supplementation can influence sleep by increasing serotonin and melatonin synthesis, but the tryptophan in a mixed meal does not typically cause immediate drowsiness.
- Tryptophan is the least abundant amino acid in proteins but plays critical structural and functional roles in bioactive peptides and therapeutic drugs.
- The indole side chain of tryptophan allows for unique chemical modifications, enabling scientists to fine-tune the properties of synthetic peptides for research and medicine.
- Late-stage functionalization techniques allow chemists to selectively modify tryptophan residues in complex peptides, creating analogs with improved stability or activity.
Demystifying the Dietary Myth of Tryptophan
The Thanksgiving Turkey Fallacy
A pervasive piece of popular folklore suggests that the tryptophan in Thanksgiving turkey is the primary reason for post-meal drowsiness. However, a closer examination of nutritional data reveals that the tryptophan content in turkey is typical for poultry. For instance, a skinless, boneless light meat turkey contains approximately 410 mg of tryptophan per pound, which is comparable to the 238 mg per pound found in chicken. Other foods, such as lamb shoulder roast, certain cheeses, and seeds like pumpkin and chia, can contain comparable or even higher concentrations of tryptophan per serving. Therefore, singling out turkey as an exceptional source of this amino acid is scientifically inaccurate.
The Biochemical Pathway to Sleep
The association between tryptophan and sleep is not entirely without merit; it is simply misunderstood in the context of a balanced meal. Tryptophan is a metabolic precursor in the synthesis of serotonin, a key neurotransmitter that regulates mood and sleep. In the brain, serotonin is subsequently converted into melatonin, a neurohormone that is critical for regulating sleep-wake cycles. Research confirms that consuming purified tryptophan supplements on an empty stomach can increase serotonin levels in the brain and decrease sleep latency (the time it takes to fall asleep). This is the factual core of the sleep connection. However, the post-Thanksgiving meal lethargy is more likely caused by the overall context of a large, carbohydrate-rich feast, which can trigger a blood sugar spike and subsequent crash, combined with the general physiological effort required for digestion.
Find out more about peptide synthesis here.
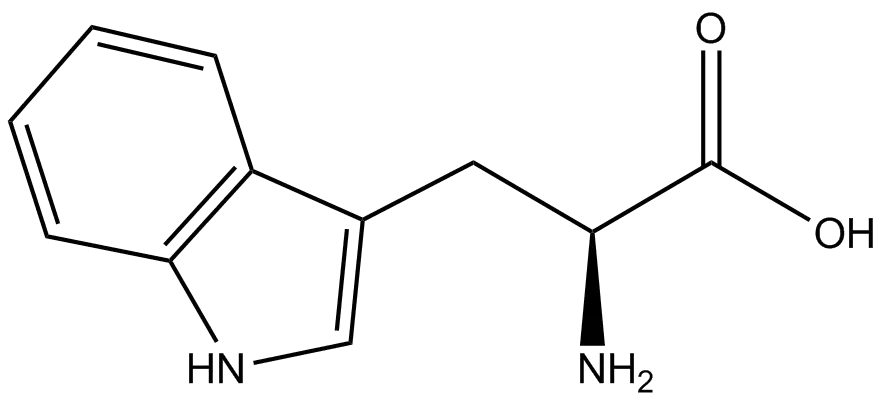
Tryptophan in Peptide Synthesis and Modification
A Scarce but Significant Amino Acid
In the realm of protein biosynthesis, tryptophan is encoded by a single codon (UGG) and is the least abundant amino acid in proteins, constituting only about 1% of their composition. Its biosynthesis is energetically costly, leading to the evolutionary principle that it is incorporated only where it provides a distinct functional or structural advantage. This low abundance but high importance extends to therapeutic peptides, where tryptophan often plays a critical role in ligand binding, enzyme catalysis, and signal transduction due to its unique chemical properties.
The Versatile Indole Ring
The functional power of tryptophan lies in its indole side chain, a large, planar, and electron-rich aromatic structure. This ring system allows tryptophan to participate in crucial cation-π interactions and π-π stacking effects, which are vital for stabilizing protein structures and mediating interactions with other molecules . Furthermore, the indole ring is relatively hydrophobic, influencing the overall solubility and folding of peptides that contain it. Predicting the solubility of tryptophan-containing peptides is a key step in research, and providers like LifeTein offer detailed protocols for dissolving hydrophobic peptides, often recommending organic solvents like DMSO or DMF for initial solubilization.
Late-Stage Functionalization for Drug Development
The unique chemical properties of the indole ring make it an excellent target for site-selective modification. Recent advances in synthetic chemistry have enabled late-stage functionalization, a powerful strategy that allows scientists to chemically modify a single tryptophan residue in a complex, fully-assembled peptide . This approach is more efficient than building the modified amino acid into the peptide from scratch.
For example, scientists have developed a catalyst-free method to add various functional groups, such as trifluoromethylthio (SCF₃) or difluoromethylthio (SCF₂H), to the tryptophan residue in a therapeutic peptide using trifluoroacetic acid (TFA) as a solvent. These modifications can significantly alter the peptide’s properties, potentially improving its metabolic stability, membrane permeability, and overall pharmacokinetic profile. These techniques are invaluable for creating diverse peptide libraries for drug discovery and for optimizing the properties of existing therapeutic peptides, a service area that companies like LifeTein specialize in through custom peptide synthesis and modification .
Find out about high-speed RUSH synthesis.
Frequently Asked Questions (FAQ)
Does eating turkey really make you sleepy?
While turkey contains tryptophan, the amount is not exceptional compared to other common meats. The drowsiness experienced after a large holiday meal is more likely due to the overall high caloric and carbohydrate intake, which diverts blood flow to the digestive system, rather than the specific effects of tryptophan from the turkey.
How does tryptophan actually affect sleep?
When consumed in isolated, high-dose supplements (1 gram or more), tryptophan can cross the blood-brain barrier and be converted into serotonin, which is then used to produce melatonin. This pathway can promote relaxation and reduce the time it takes to fall asleep. This effect is not typically produced by the tryptophan in a mixed meal.
Why is tryptophan important in peptide drugs?
Tryptophan is often found in the active sites of peptides and proteins due to its large, reactive indole ring. It is crucial for binding to targets and stabilizing structures. Modifying tryptophan residues allows researchers to create new peptide analogs with enhanced stability, activity, or other desirable drug-like properties.
What are some challenges in working with tryptophan-containing peptides?
Peptides with a high proportion of hydrophobic amino acids like tryptophan can be difficult to dissolve in aqueous solutions. LifeTein’s guidelines recommend using organic solvents like DMSO or DMF, or strong solvents like TFA, for initial solubilization of such hydrophobic peptides.
Richard, D. M., Dawes, M. A., Mathias, C. W., Acheson, A., Hill-Kapturczak, N., & Dougherty, D. M. (2009). L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. International Journal of Tryptophan Research, 2. https://doi.org/10.4137/ijtr.s2129
Xiao, Y., Zhou, H., Shi, P., Zhao, X., Liu, H., & Li, X. (2024). Clickable tryptophan modification for late-stage diversification of native peptides. Science Advances, 10(28). https://doi.org/10.1126/sciadv.adp9958
Mupparapu, N., Syed, B., Nguyen, D. N., Vo, T. H., Trujillo, A., & Elshahawi, S. I. (2024). Selective Late-Stage Functionalization of Tryptophan-Containing Peptides To Facilitate Bioorthogonal Tetrazine Ligation. Organic Letters, 26(12), 2489–2494. https://doi.org/10.1021/acs.orglett.4c00709
