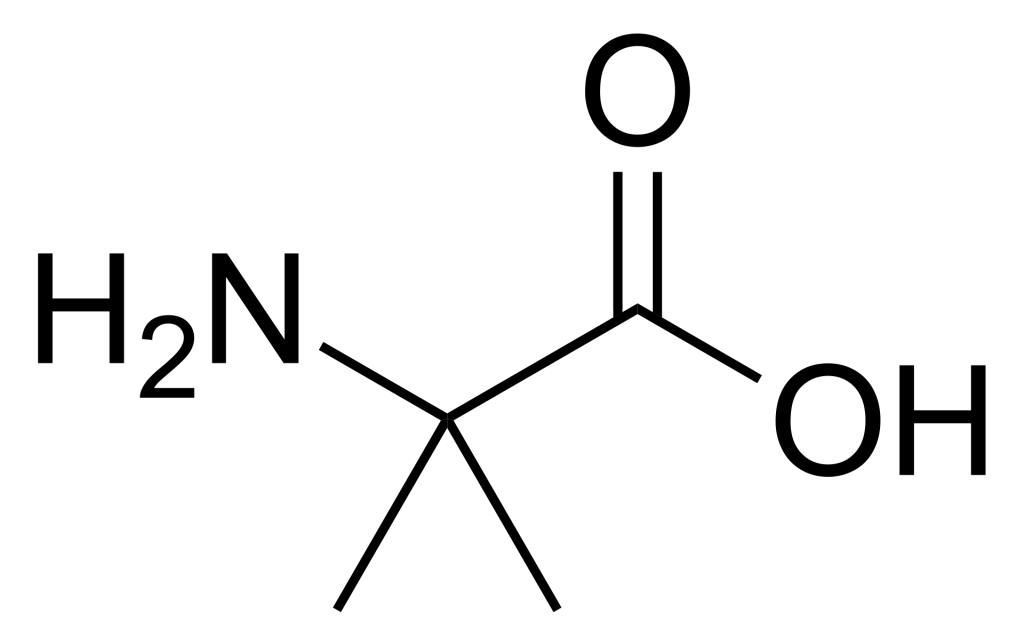
α-Aminoisobutyric acid (Aib) is a non-proteinogenic amino acid that has garnered significant attention in peptide science and medicinal chemistry due to its unique structural properties and biological applications. Unlike canonical amino acids, Aib features a gem-dimethyl group at the α-carbon, which confers exceptional conformational constraints. This characteristic makes Aib invaluable for engineering peptide stability, enhancing bioavailability, and facilitating blood-brain barrier penetration. Its incorporation into synthetic peptides often mimics natural post-translational modifications or stabilizes specific secondary structures, offering researchers a powerful tool for optimizing peptide-based therapeutics and probes.
Key Takeaways
- Aib’s gem-dimethyl group restricts conformational flexibility, promoting helical structures in peptides.
- It enhances proteolytic resistance, extending peptide half-life in vivo.
- Aib enables blood-brain barrier penetration, making it ideal for CNS-targeting therapeutics.
- Synthesis requires specialized protocols due to its non-native status and steric hindrance.
- LifeTein and other providers offer custom incorporation of Aib into peptide sequences.
Fundamentals of α-Aminoisobutyric Acid
Chemical Structure and Stereochemical Properties
Aib is characterized by a quaternary α-carbon bonded to two methyl groups, eliminating chiral centers but introducing significant steric hindrance. This structure prevents free rotation around the Cα–Cβ bond, constraining peptide backbones into right-handed 3₁₀-helical or α-helical conformations. Unlike proteinogenic amino acids, Aib lacks a side-chain functional group, reducing chemical reactivity but enhancing hydrophobic interactions. Consequently, Aib-rich peptides often exhibit increased membrane permeability and reduced conformational entropy, mimicking natural helical motifs found in antimicrobial peptides and hormones.
Natural Occurrence and Historical Context
First identified in fungal peptaibols (e.g., alamethicin), Aib is a non-coded amino acid biosynthesized via non-ribosomal pathways. Its discovery in natural antibiotics highlighted its role in stabilizing transmembrane channels and pores. Synthetic applications emerged later, leveraging Aib to engineer peptides with improved pharmacological profiles. Notably, over 120 natural peptides contain Aib, primarily from microbial sources, underscoring its evolutionary significance in molecular recognition and defense mechanisms.
Find out more about peptide synthesis here.
Functional and Biological Implications
Conformational Stabilization
The primary utility of Aib lies in its ability to induce and stabilize helical structures. In peptide design, even single substitutions with Aib can reduce conformational flexibility, minimizing unwanted aggregation or unfolding. This rigidity also mitigates entropic penalties upon target binding, improving thermodynamic efficiency.
Enhanced Metabolic Stability
α-Aminoisobutyric Acid’s quaternary carbon confers resistance to proteolytic degradation by sterically blocking access to exopeptidases and endopeptidases. Studies demonstrate that Aib-substituted peptides exhibit ~50% longer half-lives in serum compared to native sequences. This property is critical for in vivo applications where enzymatic cleavage limits therapeutic efficacy, such as in oral peptide drugs or plasma-stable probes.
Blood-Brain Barrier Penetration
Aib’s hydrophobicity and conformational constraints facilitate transcellular diffusion across biological barriers. Research shows that Aib-linked fluorescent probes (e.g., syn-bimane LASER probes) successfully traverse the blood-brain barrier (BBB), enabling CNS imaging and drug delivery. This application is pivotal for neurodegenerative disease therapeutics, where peptide-based agents often fail to achieve sufficient brain concentrations.
α-Aminoisobutyric Acid Applications in Peptide Engineering
Therapeutic Peptide Design
Aib is extensively used to optimize peptide therapeutics targeting GPCRs, ion channels, and enzymes. In diabetes research, Aib-modified glucagon-like peptide-1 (GLP-1) analogs show prolonged activity and reduced dosing frequency. Similarly, Aib-containing antimicrobial peptides (AMPs) exhibit enhanced bactericidal potency due to improved membrane integration and reduced clearance.
Fluorescent Probes and Imaging Agents
α-Aminoisobutyric Acid serves as a transporter unit for diagnostic probes, as evidenced by its role in delivering syn-bimane fluorophores across the BBB for in vivo neuronal imaging. Its incorporation into FRET peptides (e.g., those using Abz/Dnp pairs) also improves probe stability and signal-to-noise ratios in enzymatic assays.
Material Science and Self-Assembly
Aib’s helix-promoting properties enable the design of peptide nanostructures with defined geometries. These materials find applications in drug delivery scaffolds, biomimetic catalysts, and responsive hydrogels, where structural predictability is paramount.
Synthesis and Incorporation Strategies
Solid-Phase Peptide Synthesis (SPPS)
Incorporating Aib requires Fmoc- or Boc-protected derivatives compatible with standard SPPS protocols. Due to steric hindrance, coupling steps may necessitate extended reaction times or specialized activating agents (e.g., HATU). LifeTein’s expertise ensures high-efficiency incorporation, even in complex sequences involving multiple Aib residues.
Orthogonal Protection and Modification
Aib’s lack of reactive side chains simplifies synthesis but limits post-synthetic modifications. Strategies like N-terminal acetylation or C-terminal amidation are often combined with Aib incorporation to further stabilize peptides or modulate charge.
Find out about high-speed RUSH synthesis.
Frequently Asked Questions (FAQ)
What is the primary advantage of using Aib in peptides?
Aib’s gem-dimethyl group enforces helical conformations and protects against proteolysis, enhancing both stability and bioavailability.
Does Aib affect peptide immunogenicity?
Rarely. Its small, hydrophobic structure minimizes antigenic responses, making it suitable for therapeutic applications.
How does Aib improve blood-brain barrier penetration?
By increasing hydrophobicity and reducing conformational flexibility, Aib enhances passive diffusion through lipid bilayers.
Is Aib incorporation more expensive than standard amino acids?
Yes. Due to specialized synthesis and purification, Aib adds a small modification fee to peptide production costs.
Lapidot, I., Baranes, D., Pinhasov, A., Gellerman, G., Albeck, A., Grynszpan, F., & E. Shatzmiller, S. (2016). α¯ Aminoisobutyric Acid Leads a Fluorescent syn-bimane LASER Probe Across the Blood-brain Barrier. Medicinal Chemistry, 12(1), 48–53. https://doi.org/10.2174/1573406411666150518105010
