Hydroxyproline is a distinctive non-proteinogenic amino acid that serves as a critical component in the structure of collagen and has become a powerful tool in the field of peptide synthesis and design. Unlike the 20 standard amino acids directly incorporated by the ribosome, hydroxyproline is formed through a post-translational modification where proline residues within a protein chain are hydroxylated. This unique origin and its consequent structural effects make it an invaluable asset for peptide scientists aiming to control the stability, conformation, and function of synthetic peptides. Its strategic incorporation allows for the fine-tuning of peptide properties, enabling advances in biomedical research and therapeutic development.
Key Takeaways
- Hydroxyproline is a major component of collagen, providing essential stability to its triple-helical structure.
- The presence of the hydroxyl group induces conformational restraints and stereoelectronic effects that significantly influence the peptide backbone’s geometry and stability.
Chemical Fundamentals of Hydroxyproline
Structure and Discovery
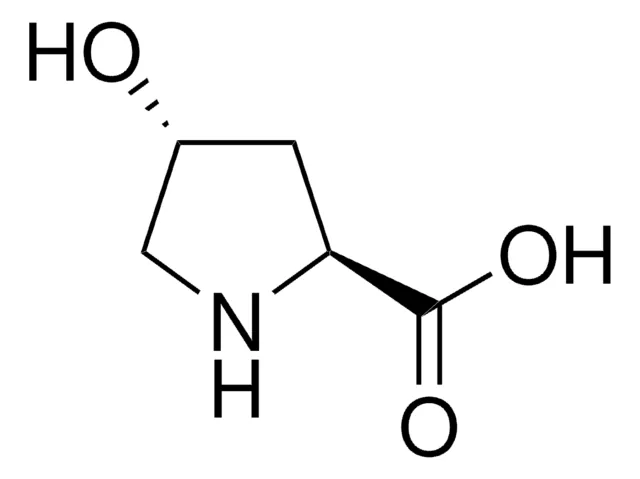
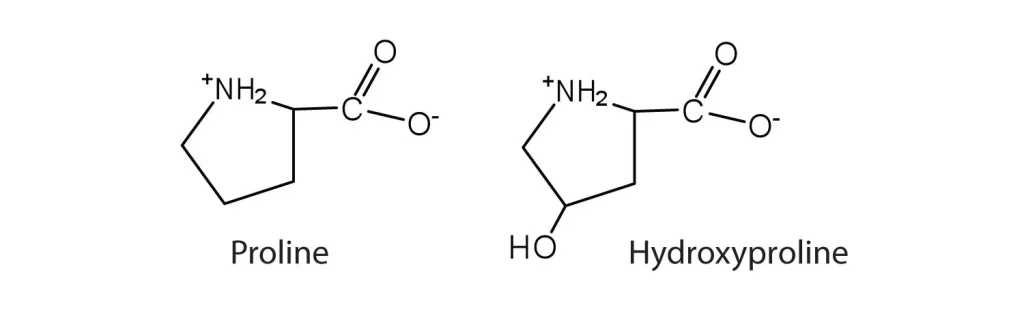
Hydroxyproline differs from its precursor, proline, by the presence of a single hydroxyl (OH) group attached to the gamma carbon atom of its pyrrolidine ring. This specific stereochemistry is crucial for its biological activity. This modification, although seemingly small, has profound implications for the physical and conformational properties of the peptides that contain it.
Biosynthesis: A Post-Translational Modification
A defining feature of hydroxyproline is that it is not directly encoded by DNA. Instead, it is manufactured within cells through the post-translational hydroxylation of proline residues that are already part of a protein chain.
The Role of Hydroxyproline in Protein Structure
Stabilization of the Collagen Triple Helix
Hydroxyproline is most renowned for its essential role in stabilizing collagen, the most abundant protein in mammals. The canonical collagen sequence features a repeating Gly-Xaa-Yaa pattern, where the proline in the Yaa position is frequently hydroxylated. This modification is not merely decorative; it is critical for the proper folding of the three polypeptide chains into a stable triple-helical structure at body temperature. The hydroxyl group on hydroxyproline stabilizes the helix primarily through stereoelectronic effects.
Find out more about peptide synthesis here.
Hydroxyproline in Peptide Synthesis and Engineering
Controlling Peptide Conformation
The strategic placement of hydroxyproline and its synthetic derivatives allows for precise control over peptide conformation. The 4-substituent on the proline ring exerts a strong influence on two key conformational equilibria: the ring pucker (endo vs. exo) and the amide bond geometry (cis vs. trans).
For instance, the natural 4(R)-hydroxyproline favors the exo ring pucker, which in turn stabilizes the trans amide bond. This knowledge can be used to pre-organize a peptide into a specific bioactive conformation, thereby enhancing its binding affinity for a target protein or its stability against degradation.
Synthetic Challenges and Solutions
The synthesis of peptides containing C-terminal proline or hydroxyproline residues presents a specific challenge: the potential formation of diketopiperazine (DKP). This side reaction occurs when the C-terminal dipeptide cyclizes, cleaving the peptide from the solid support.
Applications and Functional Implications
The ability to incorporate hydroxyproline and its derivatives into synthetic peptides opens doors to numerous advanced applications:
- Enhanced Stability: Incorporating hydroxyproline can stabilize desired secondary structures, such as turns and helices, making peptides more resistant to proteolytic degradation.
- Biophysical Studies: Peptides labeled with fluorine or other spectroscopic probes at the 4-position of proline serve as powerful tools for studying protein structure and dynamics using NMR and other techniques.
- Bioorthogonal Conjugation: Hydroxyproline-derived side chains with azide or alkyne groups allow for specific, site-selective conjugation to other molecules, such as fluorophores, lipids, or surfaces, without interfering with native functionality.
Find out about high-speed RUSH synthesis.
Frequently Asked Questions (FAQ)
Why is hydroxyproline considered an “unusual” amino acid?
Hydroxyproline is classified as “unusual” because it is not directly incorporated during protein synthesis. Instead, it is created by modifying a proline residue after the protein chain has been assembled on the ribosome, a process known as post-translational modification.
What is the primary structural difference between proline and hydroxyproline?
The key difference is structural: hydroxyproline has a hydroxyl group (-OH) attached to the gamma carbon of its pyrrolidine ring, which proline lacks. This small change has profound functional consequences, dramatically increasing the stability of collagen and influencing peptide conformation.
What special considerations are needed for synthesizing peptides with C-terminal hydroxyproline?
Peptides with proline or hydroxyproline at or near the C-terminus are susceptible to a side reaction called diketopiperazine (DKP) formation. This can be mitigated by using specialized solid-phase resins.
Can hydroxyproline be used to create stable collagen-like peptides?
Yes, absolutely. The presence of 4(R)-hydroxyproline in the Yaa position of the canonical Xaa-Yaa-Gly collagen repeat is essential for the stability of the collagen triple helix. Synthetic collagen mimetic peptides heavily rely on the incorporation of hydroxyproline to achieve a stable, native-like structure.
Ananthanarayanan, V. S. (1983). Structural Aspects of Hydroxyproline-Containing Proteins. Journal of Biomolecular Structure and Dynamics, 1(3), 843–855. https://doi.org/10.1080/07391102.1983.10507485
Pandey, A. K., Naduthambi, D., Thomas, K. M., & Zondlo, N. J. (2013). Proline Editing: A General and Practical Approach to the Synthesis of Functionally and Structurally Diverse Peptides. Analysis of Steric versus Stereoelectronic Effects of 4-Substituted Prolines on Conformation within Peptides. Journal of the American Chemical Society, 135(11), 4333–4363. https://doi.org/10.1021/ja3109664
