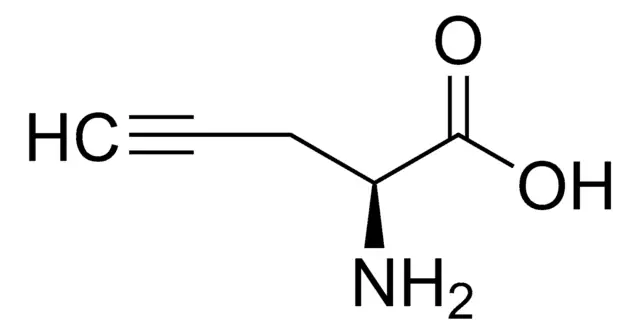
Propargylglycine (Pra) is a prominent unnatural amino acid that has become an indispensable tool in chemical biology and peptide engineering. Classified as a non-proteinogenic building block, it is not incorporated into proteins by the ribosomal machinery but is instead used synthetically to bestow novel chemical and biological properties upon peptides. Its defining feature is a terminal alkyne moiety (-C≡CH) appended to the side chain of glycine, creating a versatile handle for bioorthogonal chemistry. This simple yet powerful modification enables Pra to serve dual roles: as a potent, mechanism-based inhibitor of key enzymes in sulfur metabolism and as a unique chemical tag for precise, site-specific modification of biomolecules.
Key Takeaways
- Pra features a terminal alkyne side chain, making it a cornerstone for Cu-catalyzed azide-alkyne cycloaddition (CuAAC) “click chemistry” in peptide and protein labeling.
- It acts as a potent and irreversible inhibitor of the enzyme cystathionine γ-lyase (CSE), a key player in the endogenous production of hydrogen sulfide (H₂S).
- As an unnatural amino acid, Pra is used to expand the functional repertoire of synthetic peptides, allowing for the installation of probes, tags, and stabilizers to enhance their utility in research and drug discovery.
Fundamentals of Propargylglycine
Chemical Structure and Properties of Pra
Propargylglycine, with the molecular formula C₅H₇NO₂ and a molecular weight of 113.1 g/mol, is the simplest alkyne-bearing amino acid. Its IUPAC name is 2-amino-4-pentynoic acid. Structurally, it is an analog of the natural amino acid glycine, where one hydrogen on the alpha-carbon is replaced by a propargyl group (-CH₂-C≡CH). This L-configuration amino acid is typically supplied as a white to light-yellow crystalline powder. The presence of the terminal alkyne is central to all its applications, providing a site of unique chemical reactivity absent from the canonical 20 amino acids.
The Dual Roles of Propargylglycine (Pra)
Propargylglycine (Pra) as A Potent Biochemical Inhibitor
One of the most well-established applications of Pra, particularly its D,L-form, is as a mechanism-based enzyme inhibitor. It is a specific and potent inhibitor of cystathionine γ-lyase (CSE), with a reported half-maximal inhibitory concentration (IC₅₀) of approximately 40 µM. CSE is a pivotal enzyme in the transsulfuration pathway, responsible for the endogenous production of the gaseous signaling molecule hydrogen sulfide (H₂S). By irreversibly inhibiting CSE, Pra becomes a crucial pharmacological tool for studying the complex physiological and pathological roles of H₂S in systems ranging from cardiovascular function and neuroscience to inflammation and cancer metabolism.
Find out more about peptide synthesis here.
A Versatile Handle for Bioorthogonal Chemistry
Beyond its inhibitory function, the true power of Pra in synthetic chemistry lies in its alkyne functional group. This group participates efficiently in the Cu-catalyzed azide-alkyne cycloaddition (CuAAC), a premier example of “click chemistry”. This reaction is highly selective, proceeds rapidly under mild conditions, and is virtually inert to other biological functional groups, making it bioorthogonal. Consequently, peptides or proteins incorporating Pra can be selectively and covalently “tagged” with any molecule carrying an azide group, such as fluorescent dyes, biotin, polymers, or other peptides.
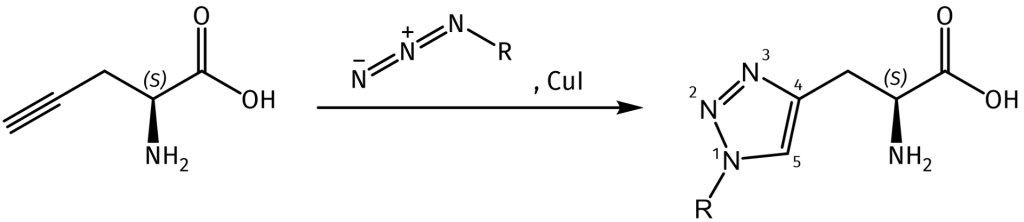
Propargylglycine (Pra) in Peptide Science and Engineering
Pra Incorporation into Synthetic Peptides
Pra is routinely incorporated into peptides using standard solid-phase peptide synthesis (SPPS) methodologies. Specialized peptide synthesis service providers can include Pra and other unnatural amino acids into custom sequences to meet specific research needs. The incorporation is straightforward, treating Pra as a unique building block during the sequential assembly of the peptide chain. This allows researchers to precisely control the location of the reactive alkyne handle within the peptide structure.
Enabling Advanced Peptide Ligand Design
A sophisticated application of Pra is in the rational design of high-affinity peptide ligands. A novel strategy involves the single-point substitution of a lysine residue with Pra within a peptide sequence. Following synthesis, the alkyne of Pra is used to attach an aminophilic salicylaldehyde tag via CuAAC. This tag can then form a reversible covalent bond with a lysine residue on the target protein’s surface. This approach has been successfully demonstrated to enhance the affinity of a peptide for its target, NEMO, showcasing a powerful method to convert standard peptides into potent, reversible-covalent binders.
Pra Advantages in Peptide Therapeutic Optimization
The use of unnatural amino acids like Pra aligns with broader strategies in peptide drug discovery to overcome inherent limitations of natural peptides, such as poor metabolic stability and low membrane permeability. Introducing Pra can modulate a peptide’s physicochemical properties, alter its conformation, and provide a direct avenue for conjugation to half-life extending polymers (like PEG) or drug carriers. Furthermore, the alkyne handle can be used to install environment-sensitive fluorophores for imaging or tracking, as noted in studies with cystine knot peptides.
Find out about high-speed RUSH synthesis.
Frequently Asked Questions (FAQ)
What makes Propargylglycine an “unusual” or “unnatural” amino acid?
Propargylglycine is classified as unnatural or non-proteinogenic because its structure, featuring an alkyne side chain, does not exist among the 20 standard amino acids encoded by DNA and used in natural ribosomal protein synthesis. It is a product of synthetic chemistry designed to provide functionalities not available in nature.
Is Pra’s primary use as an inhibitor or a chemical handle?
It serves both critical functions, and the primary use depends on the research context. In physiological and pharmacological studies, its role as a CSE inhibitor to probe H₂S biology is paramount. In chemical biology, biotechnology, and drug discovery, its utility as a bioorthogonal click chemistry handle for labeling and conjugating biomolecules is its key asset.
How is Pra incorporated into peptides?
Pra is incorporated synthetically. The most common method is solid-phase peptide synthesis, where it is used as a protected amino acid building block and coupled in sequence like any standard amino acid. For producing more complex proteins, advanced techniques like genetic code expansion in live cells can be used to incorporate Pra site-specifically.
Can Pra be purchased for research?
Yes, L-Propargylglycine is commercially available from fine chemical and biochemical suppliers. It is typically sold as a high-purity (e.g., ≥98%) powder for research use.
Adhikari, A., Bhattarai, B. R., Aryal, A., Thapa, N., KC, P., Adhikari, A., Maharjan, S., Chanda, P. B., Regmi, B. P., & Parajuli, N. (2021). Reprogramming natural proteins using unnatural amino acids. RSC Advances, 11(60), 38126–38145. https://doi.org/10.1039/d1ra07028b
Sharma, K. K., Sharma, K., Rao, K., Sharma, A., Rathod, G. K., Aaghaz, S., Sehra, N., Parmar, R., VanVeller, B., & Jain, R. (2024). Unnatural Amino Acids: Strategies, Designs, and Applications in Medicinal Chemistry and Drug Discovery. Journal of Medicinal Chemistry, 67(22), 19932–19965. https://doi.org/10.1021/acs.jmedchem.4c00110
