Please click here to receive a quote now!
Incomplete deprotection and amino acid-coupling reactions can complicate certain processes. Longer reaction times and the use of increased strength reagent could solve this problem. However, in some extreme cases, these methods are insufficient and the protecting group cannot be removed efficiently. In addition, the side chains of some sequences render them prone to self-association by hydrogen bonding. This leads to aggregation and the formation of beta-sheet-like secondary structures that can halt peptide synthesis.
LifeTein's optimized protocol and PeptideSyn technology provide a method for circumventing the problems associated with difficult sequences. This technology can change peptide structure by protecting some of the peptide amide bonds, giving rise to peptides that contain tertiary amides at specific intervals. This leads to better preservation of the peptide chain, and more efficient deprotection and coupling reactions.
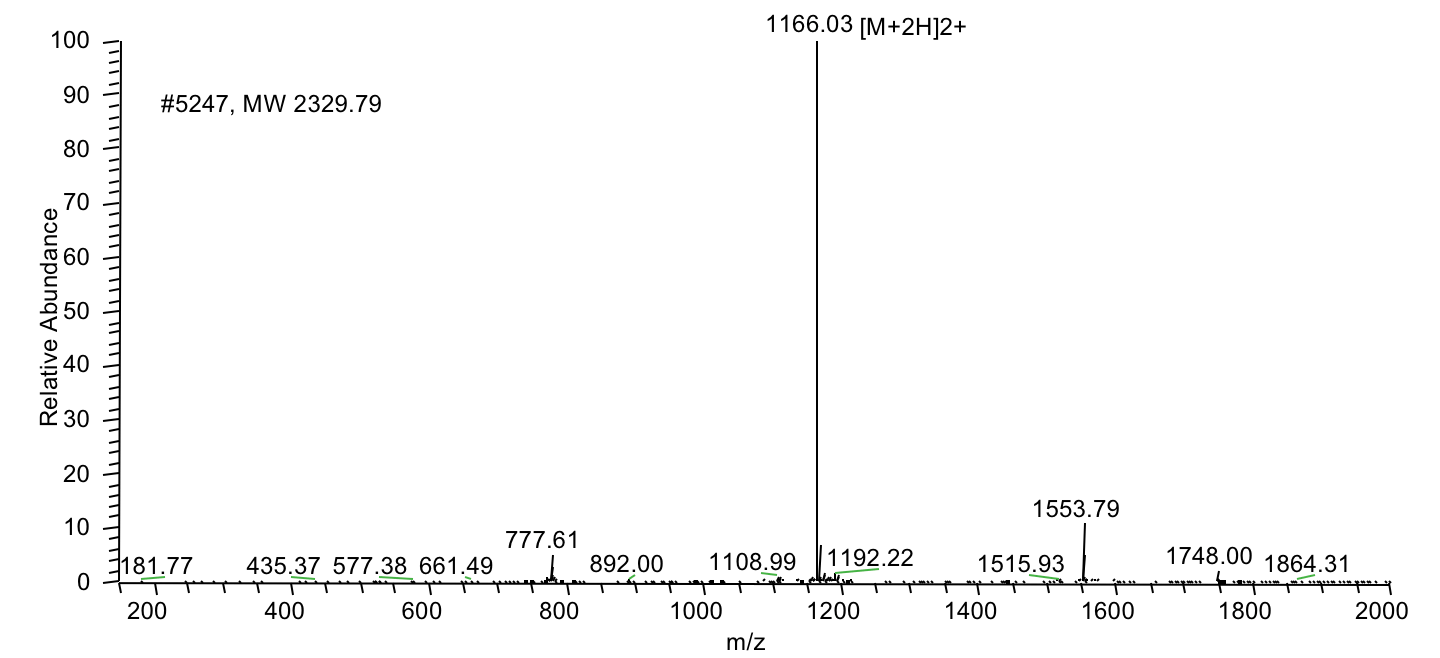
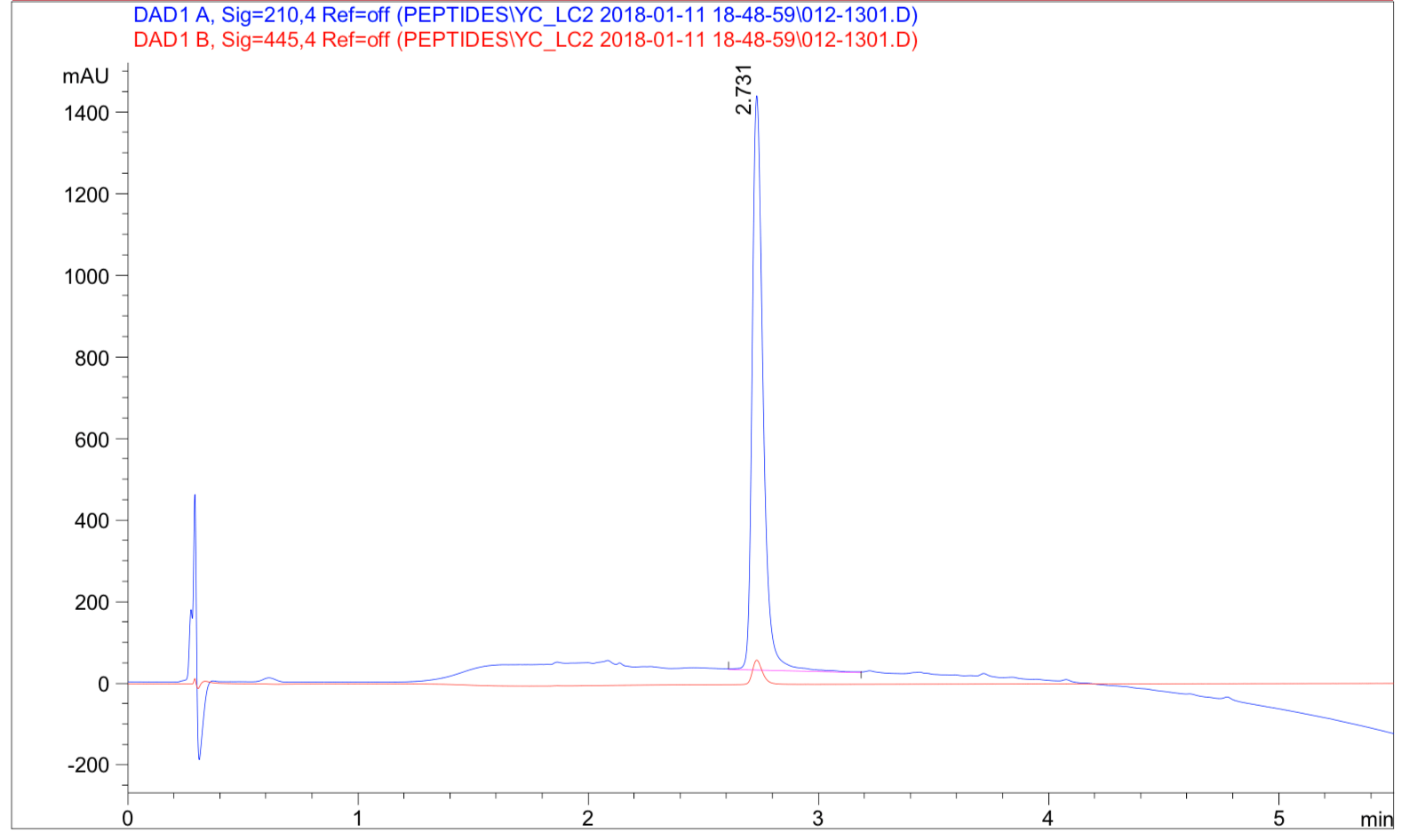
Case 1: Solid-phase synthesis of a peptide with four phosphorylation sites.
A 14 amino acid peptide (molecular weight: 1981.53) with four phosphorylation sites was synthesized at 95% purity and delivered in 3 weeks: ABC-pSpT-ABC-pS-ABC-pT-x
HPLC Results:
MS Results:
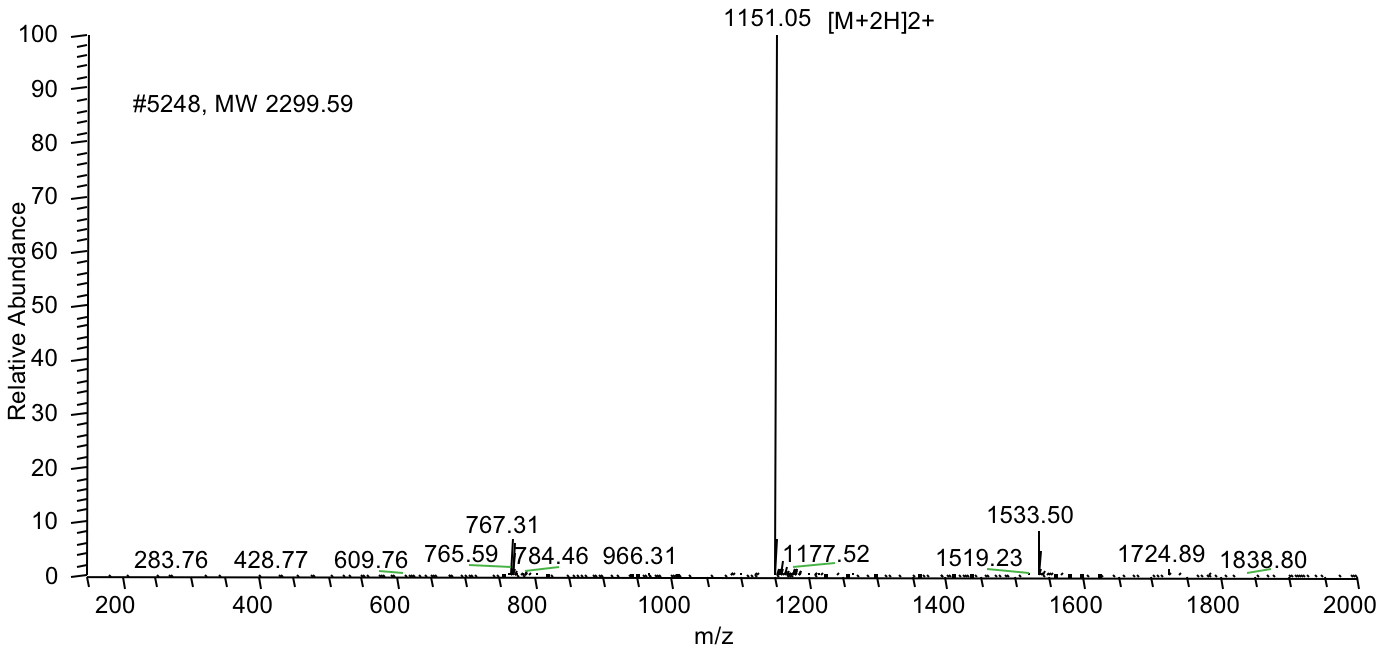
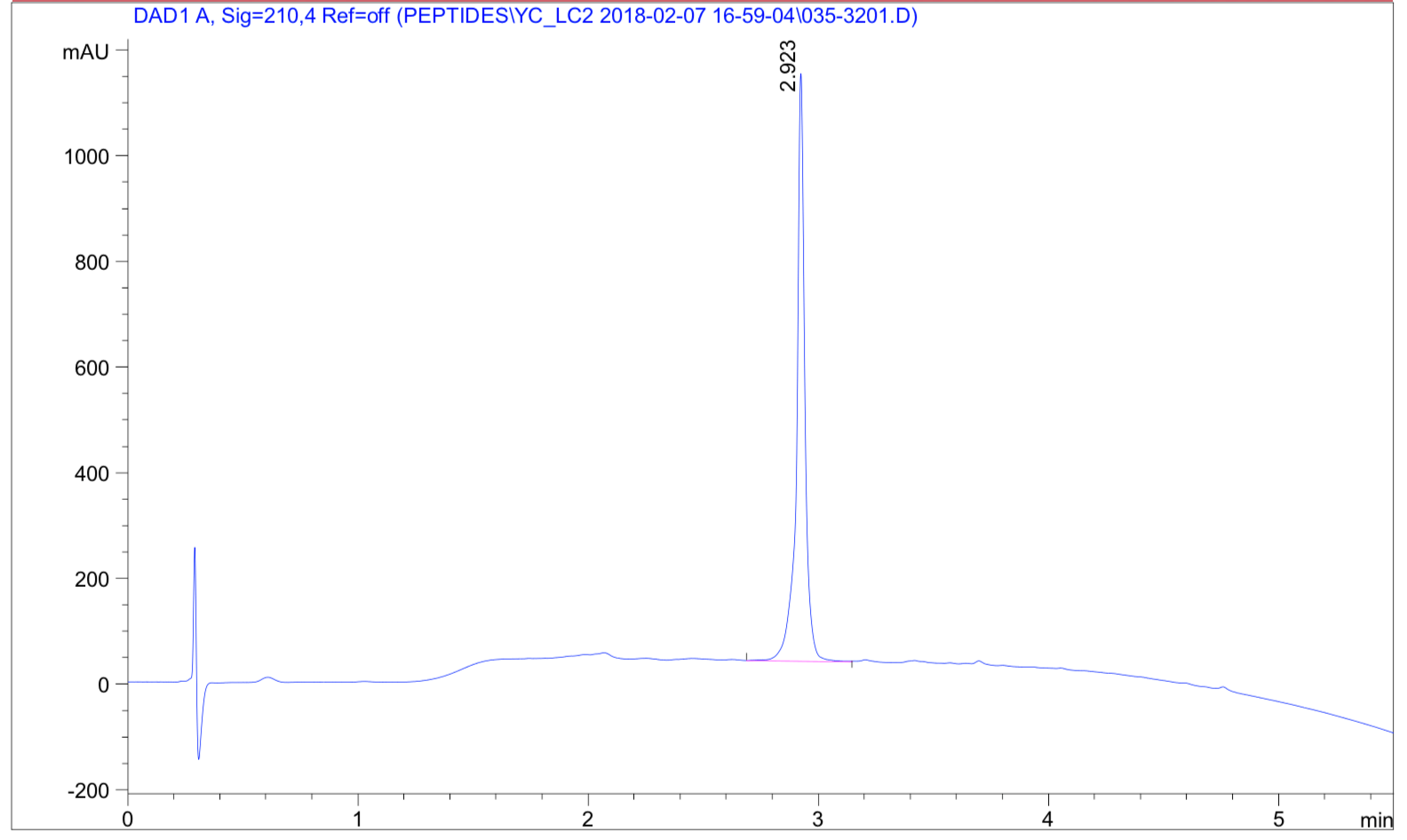
Case 2: Long peptide synthesis is always difficult and challenging. Recently, a 169-amino-acid peptide was successfully synthesized in 4 weeks. The following case is a very hydrophobic 68 amino acid peptide (85% purity) with a FITC modification at the N terminus. The peptide was successfully synthesized in 4 weeks.
HPLC Results:
MS Results: